Draw A Bohr Model
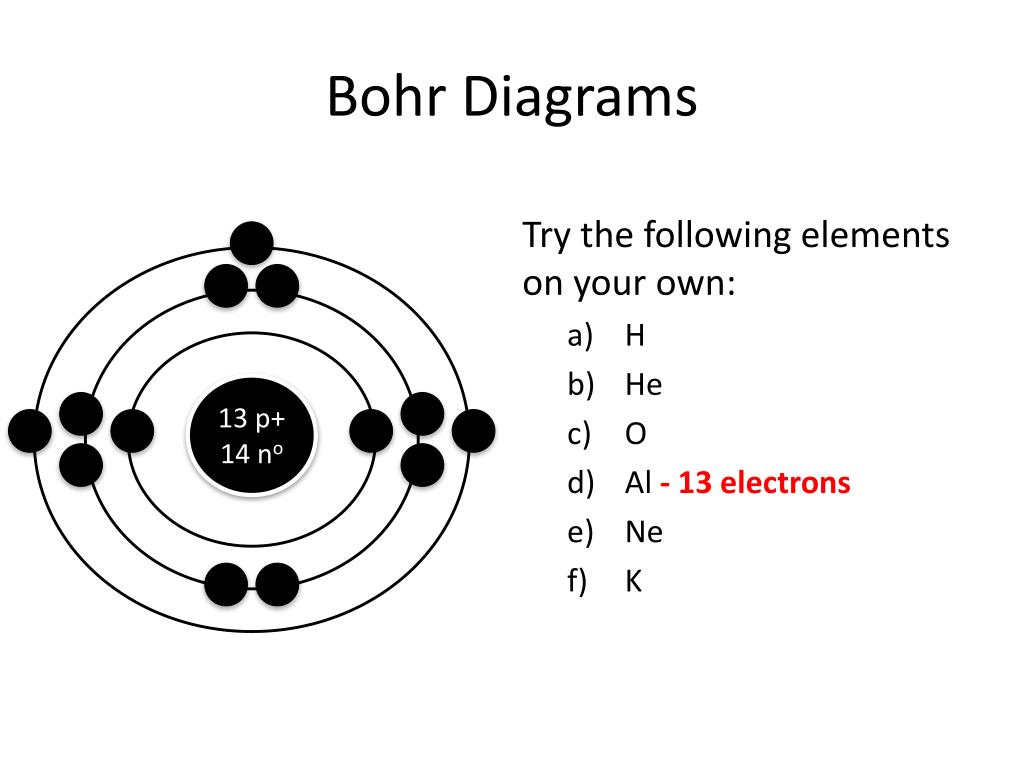
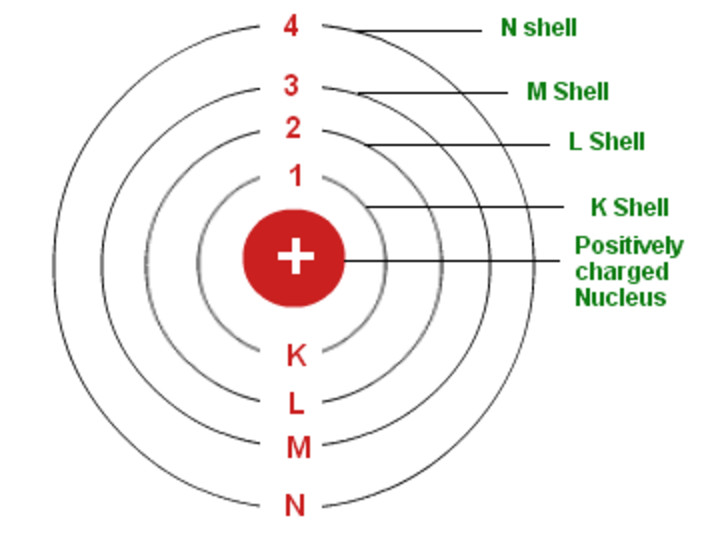
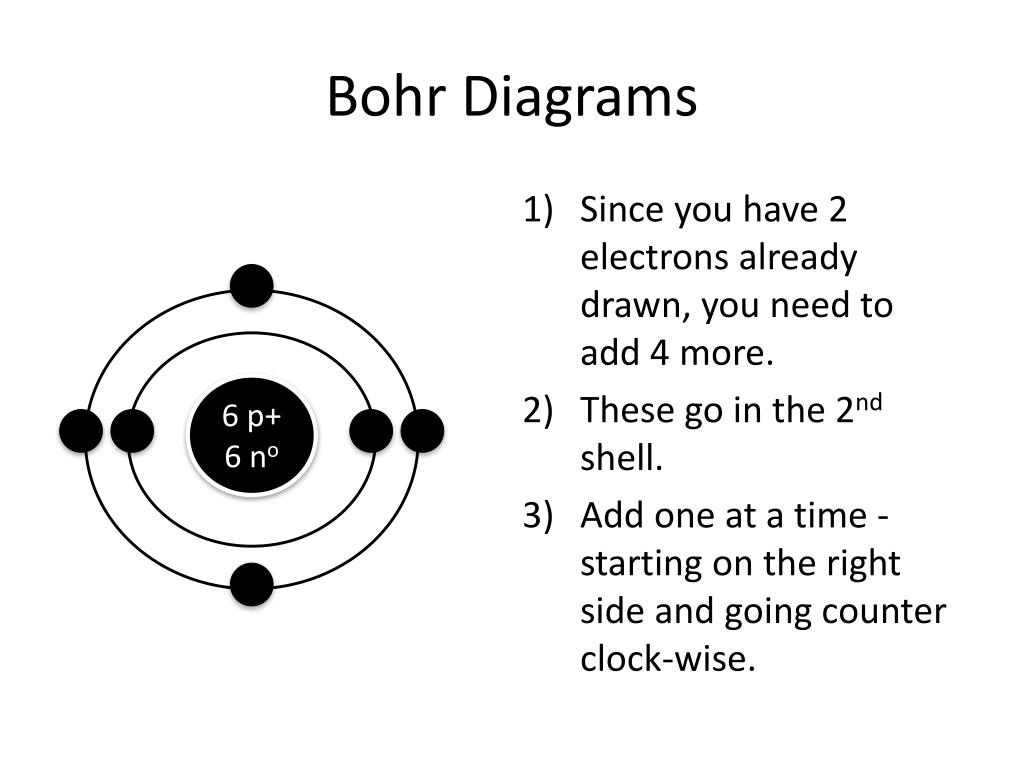
Draw A Bohr Model - Find the number of protons, electrons, and neutrons of an atom. Draw the second electron shell,. Nobel prize in physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Web how to draw bohr model of an atom? Get these slides and the guided notes sheets: Bohr’s model required only one assumption: Web bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr. Web bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. The boron family is located in the 13 th group of the periodic table: You will also get the hd images of the periodic table (for free). Draw shells around the nucleus to represent the orbitals. • the atomic number of boron is 5. Learn how to draw a. Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. The maximum number of electrons that can fill each shell is: • the atomic number of boron is 5. Web bohr's model calculated the following energies for an electron in the shell, n. Electrons must occupy the lowest available shell, closest to the nucleus. Web and find protons, neutrons, and electrons from a periodic table card. Learn how to draw a. Draw the second electron shell,. Web i'll teach you how to figure out the number of electron energy levels and the electrons that fit in each using the periodic table. Web the bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. Web drawing bohr models. The steps to drawing bohr diagrams for. Learn how to draw a. Nobel prize in physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Draw shells around the nucleus to represent the orbitals. • the atomic number of boron is 5. You can effortlessly find every single detail about the elements from this single interactive periodic table. The bohr model of the atom was proposed by neil bohr in 1915. Two in the first shell, eight in the second shell, eight in the third shell. Bohr’s model required only one assumption: E ( n) = − 1 n 2 ⋅ 13.6 ev. Learn how to draw a. Draw the nucleus of an atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. Web and find protons, neutrons, and electrons from a periodic table card. Web bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of. Draw the electrons in their respective orbitals. You can effortlessly find every single detail about the elements from this single interactive periodic table. Two in the first shell, eight in the second shell, eight in the third shell. Electrons must occupy the lowest available shell, closest to the nucleus. You will get the detailed information about the periodic table which. Draw the second electron shell,. Web drawing bohr models. Web and find protons, neutrons, and electrons from a periodic table card. Web bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. It came into existence with the modification of rutherford’s model of an atom. Find the number of protons, electrons, and neutrons of an atom. Web bohr model energy levels. The bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. • the atomic number of boron is 5. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Draw the first shell add the electrons, the first shell can only hold 2 5. Draw the next shell if you have more electrons to add 6. Bohr explained the hydrogen spectrum in terms of electrons. Information derived from the boron box: Updated on january 27, 2020. Bohr used the formula 2n 2 to calculate maximum number of electrons that could be housed in an energy level or shell. Draw the second electron shell,. Web 31k views 8 years ago. Draw the first shell 4. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. Calculating electron energy for levels n=1 to 3. The bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Web the bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. Nobel prize in physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Although not completely correct, the bohr model can give us a visual representation of electronic levels in atoms. Draw the nucleus of an atom. Web what is bohr’s model of an atom? Draw the electrons in their respective orbitals.
How To Draw A BohrRutherford Diagram YouTube
Bohr Model of the Atom Overview and Examples
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download

Potassium Bohr Model Diagram, Steps To Draw Techiescientist
Bohr's Model of an Atom Chemistry, Class 11, Structure of Atom

Carbon Bohr Model — Diagram, Steps to Draw Techiescientist
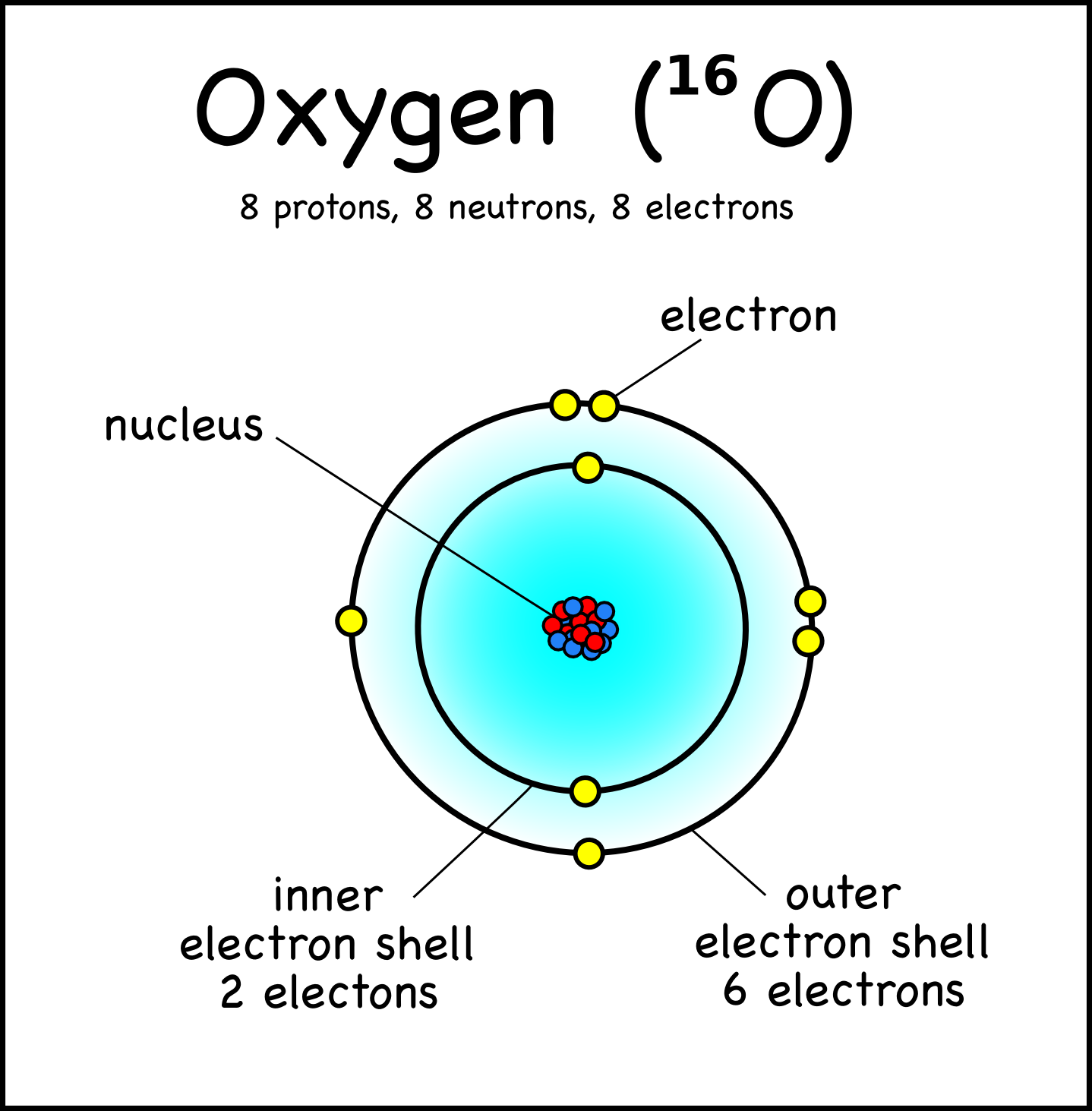
Bohr Model Drawing Of Oxygen at GetDrawings Free download
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
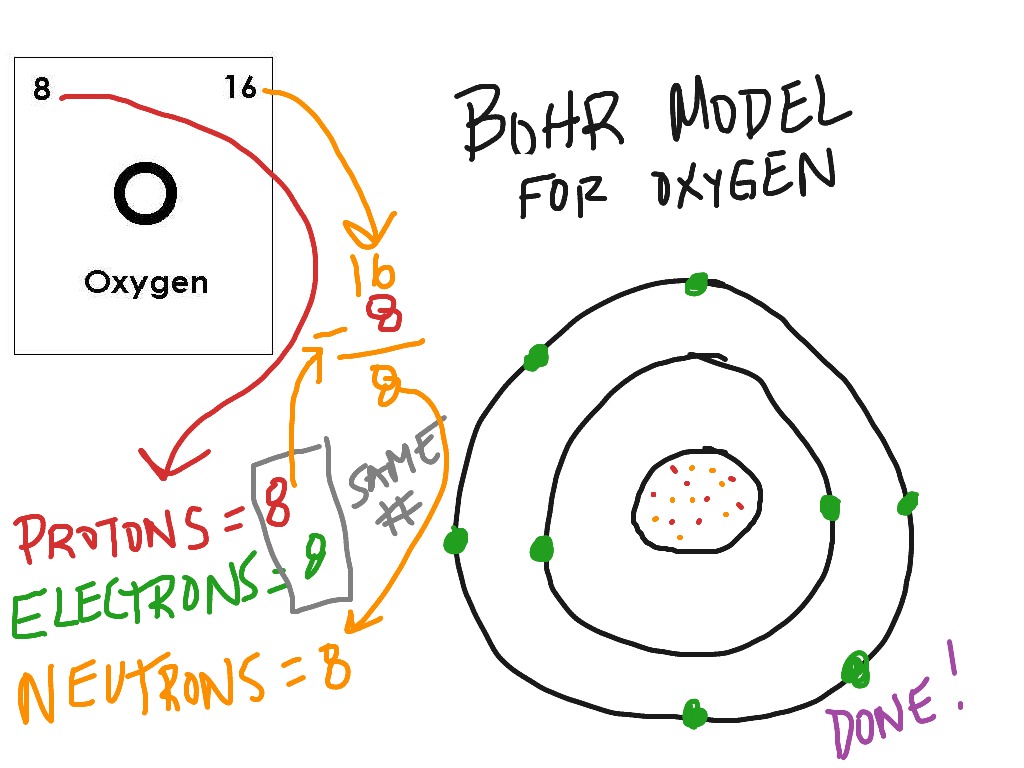
how to draw a bohr model

Bohr Model Drawing Of Oxygen at GetDrawings Free download
You Will Also Learn How To Write Bohr Model Electron Configuration.
Web And Find Protons, Neutrons, And Electrons From A Periodic Table Card.
Web Bohr's Model Suggests That The Atomic Spectra Of Atoms Is Produced By Electrons Gaining Energy From Some Source, Jumping Up To A Higher Energy Level, Then Immediately Dropping Back To A Lower Energy Level And Emitting The Energy Different Between The Two Energy Levels.
• The Atomic Number Of Boron Is 5.
Related Post: