Draw A Lewis Structure For C2H6O
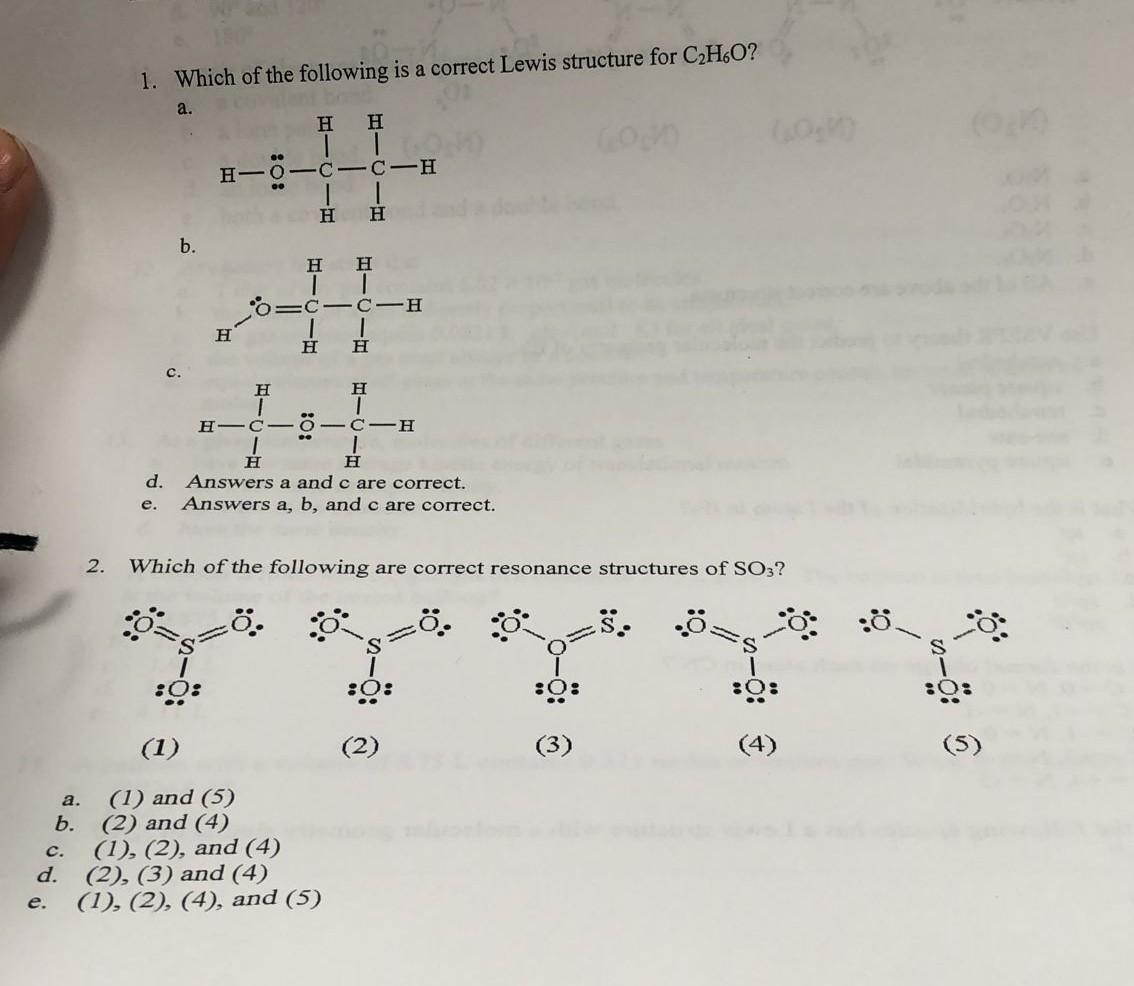
Draw A Lewis Structure For C2H6O - The individual atoms with all their valence electrons are shown in this structure to know the bond formation, molecular geometry, and shape of the molecule. Ethanol, and dimethyl ether.the fact that there are no double bonds or rings makes this very simple.check me. Three are two ways to draw the lewis structure for c2h6o. And then hydrogen, group 1, one valence electron; Web drawing lewis structures for molecules with one central atom: Find more chemistry widgets in wolfram|alpha. Every atom tends to complete its octet ( 8 electrons) either by gaining or losing electrons except hydrogen and helium as they complete their duplet. In this video you will learn how to draw lewis structure dimethyl ether c2h6o. Draw lewis structure of both isomers. Predict the geometry at each carbon atom and at the oxygen atom. Predict the geometry at each carbon atom and at the oxygen atom. Web lewis structure finder added jun 9, 2014 by webtester in chemistry this widget gets the lewis structure of chemical compounds. Web first, we need to draw the lewis structures for the two isomers of c2h6o. Find more chemistry widgets in wolfram|alpha. Web how to draw the lewis. The two carbon atoms (c) are at the center and they are surrounded by 3 hydrogen atoms (h). So let's multiply that times 2. How to draw a lewis structure for ethanol? Let’s draw and understand this lewis dot structure step by step. The two isomers are ethanol (c2h5oh) and dimethyl ether (ch3och3). This is called dimethyl ether. Web chemistry questions and answers. The individual atoms with all their valence electrons are shown in this structure to know the bond formation, molecular geometry, and shape of the molecule. Lewis structure is a 2d representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. Web let's do. So let's multiply that times 2. Web chemistry chemistry questions and answers draw a lewis structure for c2h6o in which o is bonded to two kinds of atoms. Put the least electronegative atom in the center. On the periodic table, carbon is in group 4 or 14, so it has 4 valence electrons, but we have 2 of them. The. Web how to draw the lewis dot structure for c2h6: How to draw a lewis structure for ethanol? The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. In this video you will learn how to draw lewis structure dimethyl ether c2h6o. The individual atoms with all their valence. Web master how to use organic chemistry to make lewis structures easier. Web let's do the lewis structure for c2h6, ethane. On the right, the oxygen atom's on the outside with the. Web chemistry chemistry questions and answers draw a lewis structure for c2h6o in which o is bonded to two kinds of atoms. Do not consider cyclic (ring) structures. Lewis structure is a 2d representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. In this video you will learn how to draw lewis structure dimethyl ether c2h6o. Web draw lewis structure for dimethyl ether c2h6o. Find the total valence electrons for the c2h6 molecule. Web let's do the lewis structure for. Lewis structure is a 2d representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. Predict the geometry at each carbon atom and at the oxygen atom. Draw a lewis structure for dmf, identify all central atoms and state the geometry at each central atom. Ethanol, and dimethyl ether.the fact that there are. Both use all 20 valence electrons and achieve full outer. 413 views 2 years ago chemistry. And then hydrogen, group 1, one valence electron; Web c2h6 lewis structure. The two isomers are ethanol (c2h5oh) and dimethyl ether (ch3och3). Find more chemistry widgets in wolfram|alpha. 413 views 2 years ago chemistry. How to draw a lewis structure for ethanol? Draw a lewis structure for ethyl alcohol, c2h60. Web drawing lewis structures for molecules with one central atom: The two isomers are ethanol (c2h5oh) and dimethyl ether (ch3och3). On the left, we have the oxygen atom between the two carbons. There are only two isomers of c2h6o: Lewis structure helps with understanding the placement of atoms in the structure along with its valence electrons. 413 views 2 years ago chemistry. Draw lewis structure of both isomers. Let’s draw and understand this lewis dot structure step by step. Use your diagram to answer the following questions. Draw three lewis structures for c 3 h 8 o \mathrm{c}_3 \mathrm{h}_8 \mathrm{o} c 3 h 8 o. The structural formula of dimethylformamide (dmf), an industrial solvent, is shown. Every atom tends to complete its octet ( 8 electrons) either by gaining or losing electrons except hydrogen and helium as they complete their duplet. Lewis structure is a 2d representation of the compound, which represents only the valance shell electrons of the atoms in the molecule. Web 0:00 / 3:44 how to draw a lewis structure for dimethyl ether c2h6o? With a bite sized video explanation from johnny betancourt. It is based on the octet rule i.e. Draw a lewis structure for ethyl alcohol, c2h60.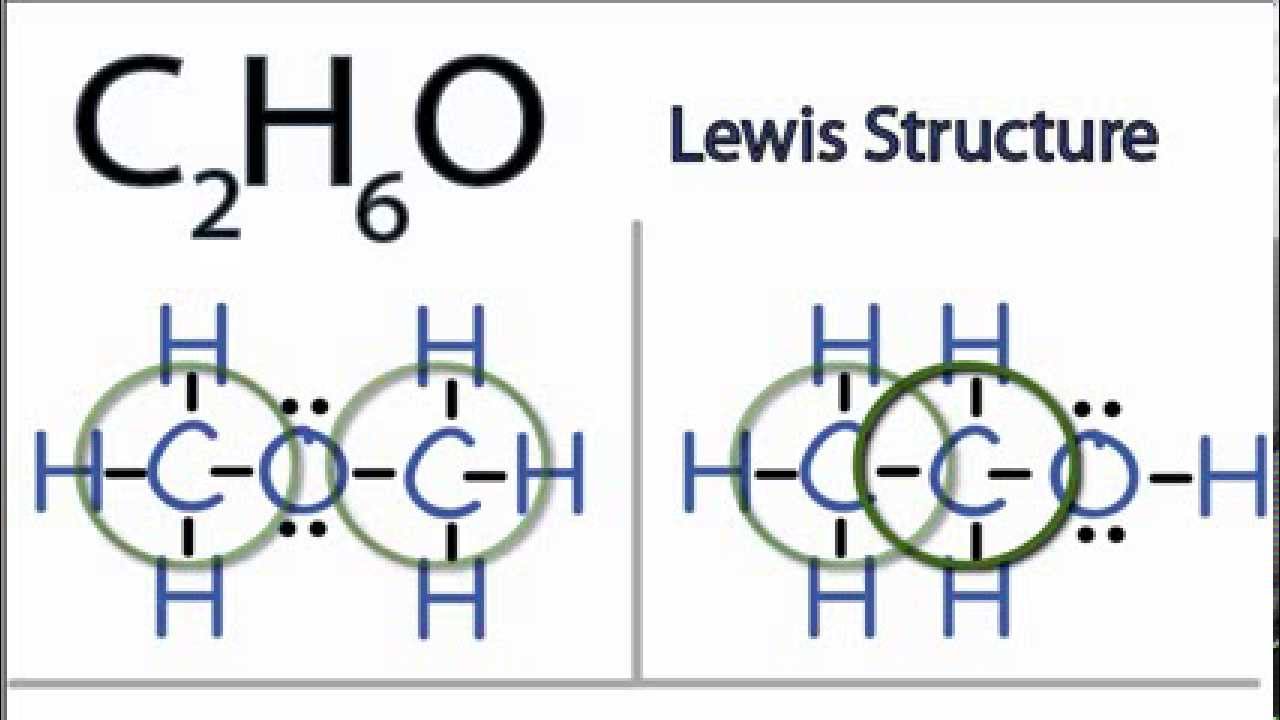
C2H6O Lewis Structure How to Draw the Lewis Structure for C2H6O YouTube
Ethanol, Atomic Formula c2h6o, Elements Found in Molecul...

C2h6o Lewis Structure
C2h6o Lewis Structure
C2h6o Lewis Structure
C2h6o Lewis Structure

C2H6O Lewis Structure (Dimethyl Ether) YouTube

C2h6o Lewis Structure
C2h6o Lewis Structure

C2h6o Lewis Structure
Find The Total Valence Electrons For The C2H6 Molecule.
Send Feedback | Visit Wolfram|Alpha Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Three Are Two Ways To Draw The Lewis Structure For C2H6O.
On The Right, The Oxygen Atom's On The Outside With The.
Related Post: