Draw A Lewis Structure For Hcn
Draw A Lewis Structure For Hcn - Does this molecule exhibit resonance? The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for hydrogen cyanide (hcn). At least two lewis structures can be drawn for bcl 3. 3.5k views 6 years ago chem 101: Web the lewis structure of hcn shows a triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Web 6 steps to draw the lewis structure of hcn step #1: • how to draw lewis. Does this molecule exhibit resonance? Enjoy 2 weeks of live tv, on us stream more, watch easier, and spend less with youtube tv. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Draw at least one other lewis structure for the nitrate ion that is not plausible based on formal charges. A triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Here, the given molecule is. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Nov 12, 2017 here's how to do it. Web write lewis symbols for neutral atoms and ions. We'll also compare hnc to hcn and discuss why both are of inter. Put least electronegative atom in. A triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. In order to draw the lewis. Web use these steps to correctly draw the hcn lewis. Put one electron pair in each bond4. Put least electronegative atom in centre3. Web science chemistry chemistry questions and answers draw the lewis structure for hcn. Draw lewis structures depicting the bonding in simple molecules. Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen. Does this molecule exhibit resonance? #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable lewis structure Here we have. A triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Web to draw the lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. Make sure you put the correct atom at the center of the hcn molecule. 📌you can draw. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Step method to draw the lewis structure of hcn. This problem has been solved! In this method, we find the bonds and lone pairs for the whole molecule, then plug it in to the atoms that we have to. A) ccl4 b) n2h2 c) hcn 6) a) draw the molecular orbital diagram of the given compounds. Web to draw the lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. Does this molecule exhibit resonance? Web learn the steps to draw the lewis structure of hcn in just 1. Put least electronegative atom in centre3. Counting valence electrons before we can start drawing the lewis structure, we need to determine the total. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Does this molecule exhibit resonance? To know the valence electrons of hcn, let us go through the valence. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. 3.5k views 6 years ago chem 101: Enjoy 2 weeks of live tv, on us stream more, watch easier, and spend less with youtube tv. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.. Chemistry 1 answer ernest z. Determining the formal charge formal charge is a vital concept in drawing lewis structures. Web the lewis structure of hcn shows a triple bond between the carbon and nitrogen atoms, with a lone pair of electrons on the nitrogen atom. Web learn the steps to draw the lewis structure of hcn in just 1 minute. 5) for the below compounds, draw the lewis structure, indicate the formal charge on each atom, determine the hybridization of all interior atoms, show the hybridization scheme, and sketch the orbital overlapping based on valance bond theory. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web how to draw lewis structure for hcn? Web use these steps to correctly draw the hcn lewis structure: Draw lewis structures depicting the bonding in simple molecules. Enjoy 2 weeks of live tv, on us stream more, watch easier, and spend less with youtube tv. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. (1 × 1) + (4 × 1) + (5. After determining how many valence electrons there are. At least two lewis structures can be drawn for bcl 3. Web to draw the lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. Draw a skeleton structure put the least electronegative atom c in the middle with h and cl on either side.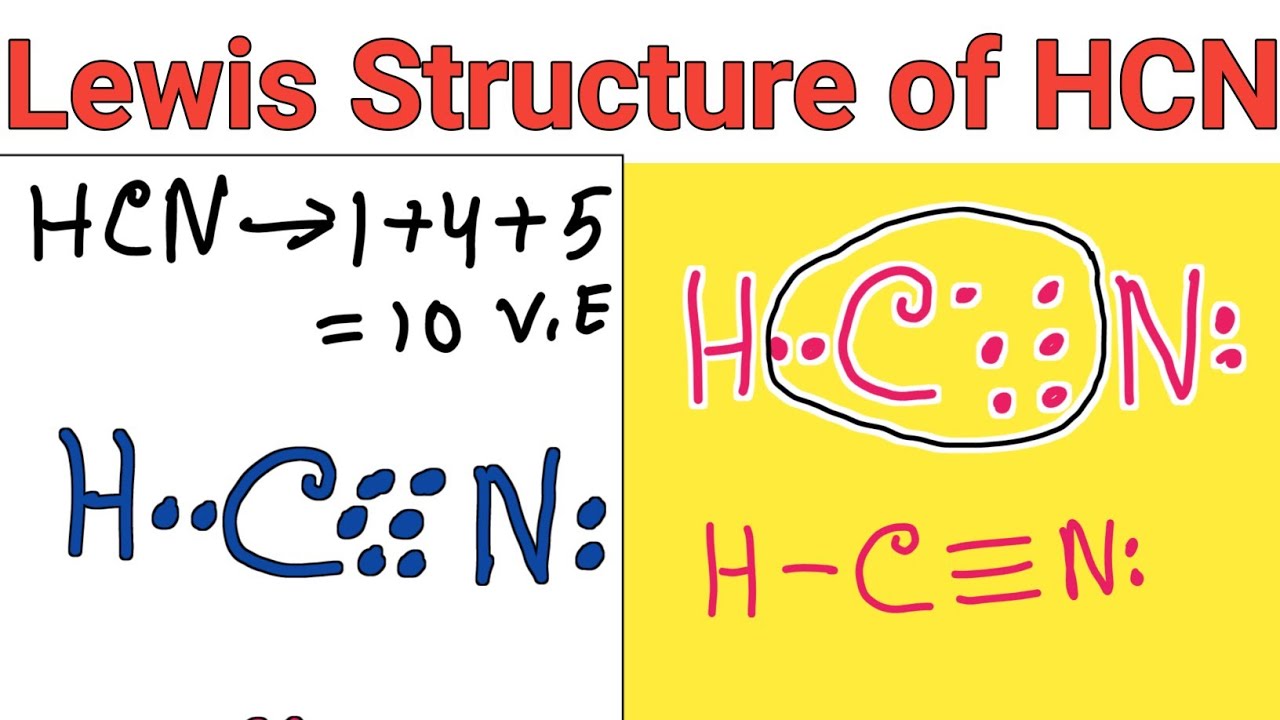
Lewis structure of HCN (Hydrogen cyanide) YouTube
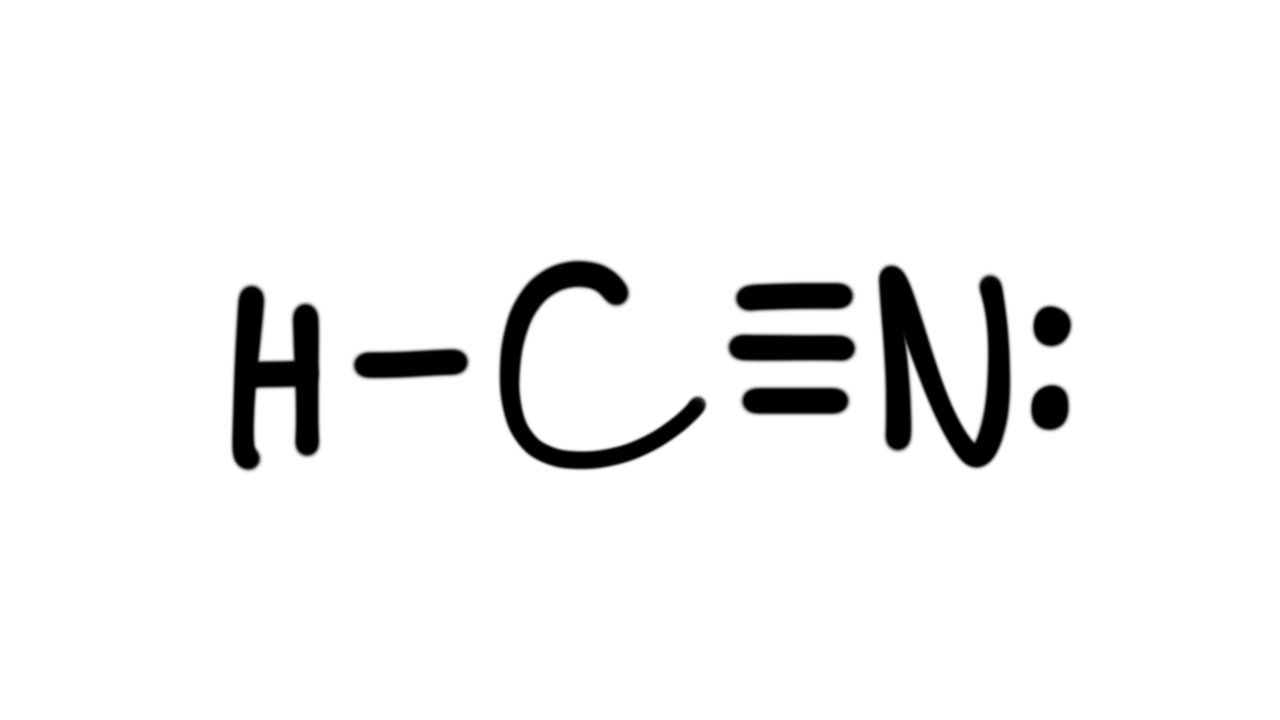
Lewis Diagram For Hcn

How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw

Hcn Lewis Structure Bonds Draw Easy

HCN Lewis Structure (Hydrogen Cyanide) Molecules, Chemical formula, Lewis
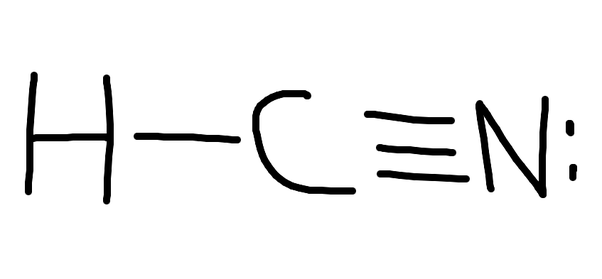
Lewis Diagram For Hcn
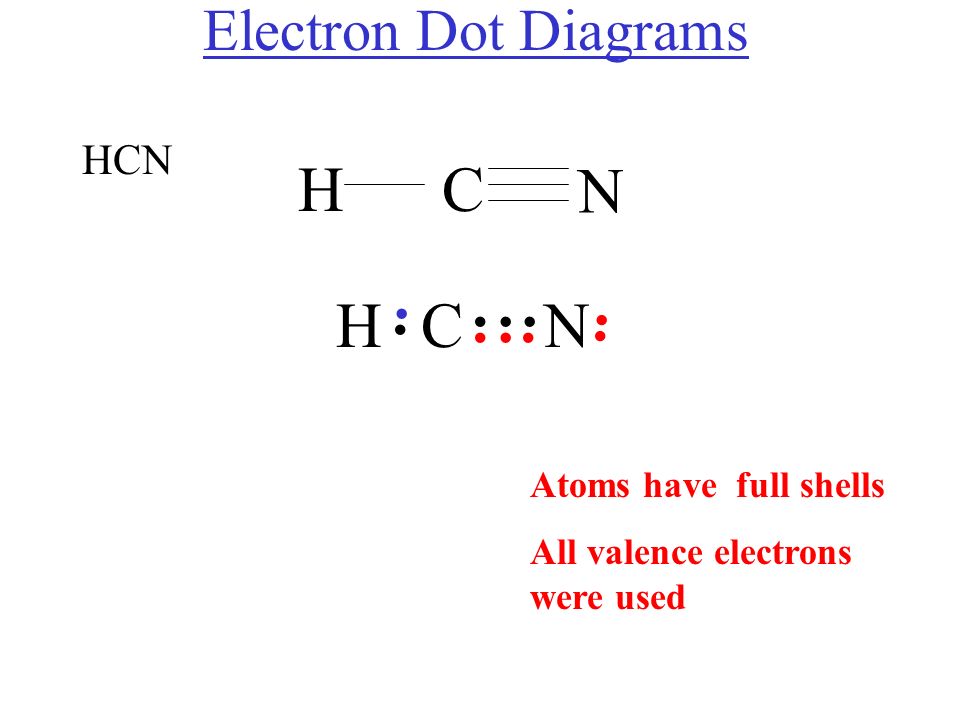
Lewis Diagram For Hcn

Lewis Dot Diagram Of Hcn

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
#1 First Draw A Rough Sketch #2 Mark Lone Pairs On The Atoms #3 Calculate And Mark Formal Charges On The Atoms, If Required #4 Convert Lone Pairs Of The Atoms, And Minimize Formal Charges #5 Repeat Step 4 If Needed, Until All Charges Are Minimized, To Get A Stable Lewis Structure
3.5K Views 6 Years Ago Chem 101:
Here, The Given Molecule Is Hcn.
Select The Center Atom (H Is Always Outside).
Related Post: