Draw An Appropriate Lewis Structure For Ch2Chch3
Draw An Appropriate Lewis Structure For Ch2Chch3 - The number of atoms in the molecule. Web for ch2chch3 draw an appropriate lewis structure. We need to draw the lewis structure for the molecule where we have carbon, it's only attached to 1 hydrogen, then it's attached to a second carbon and that second carbon looks like it's attached to a third carbon. Draw an appropriate lewis structure for ch2chch3. The types of atoms in the molecule. Include all lone pairs of. This is a dead giveaway that n carries a +1 charge (and no lone pairs). Web lewis structure are the diagrams, which represent the bonding between atoms of a molecule. Assign an ax m e n designation; There are 2 steps to solve this one. Web drawing lewis structures for molecules with one central atom: There are 2 steps to solve this one. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. This is a dead giveaway that n carries a +1 charge (and no lone pairs). How many sigma and pi bonds does it contain? Ch3och3 has its use as propellants in aerosol products like hair sprays. View the full answer step 2 step 3 final answer previous question next question transcribed image text: Web for ch2chch3 draw an appropriate lewis structure. Here’s the best way to solve it. The types of atoms in the molecule. In liquid form, it is volatile and poisonous. Web drawing lewis structures for molecules with one central atom: The length of the bonds. First, lets find the how many valence electrons chlorate has: The propene lewis structure shows that it has 8 sigma and 1 pi bonds. There are 2 steps to solve this one. Electrons are shown as dots or for bonding electrons as a line between the two atoms. Next lets draw the basic framework of the molecule: Draw an appropriate lewis structure for chechch draw the molecule by placing atoms on the grid and connecting them with bonds. Figure out how many electrons the. Web in this example, we draw the lewis structure for the organic molecule ch3cooh, acetic acid, and evaluate it using formal charge. How many sigma and pi bonds does it contain? Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. There are 26 valence electrons. A lewis structure is a. Draw the lewis dot structure for sf6 and provide the following information. Here’s the best way to solve it. For $\ce{ch2chch3}$ draw an appropriate lewis structure. Web in this example, we draw the lewis structure for the organic molecule ch3cooh, acetic acid, and evaluate it using formal charge. 1.2.1 lewis structure of diatomic molecules. Web in this example, we draw the lewis structure for the organic molecule ch3cooh, acetic acid, and evaluate it using formal charge. For each compound, draw an appropriate lewis structure, fully describe the bonding and geometry using a valence bond approach, determine whether the molecule is polar, and include a sketch of the molecule. Web the following drawing shows how. Web for ch2chch3 draw an appropriate lewis structure. For each compound, draw an appropriate lewis structure, fully describe the bonding and geometry using a valence bond approach, determine whether the molecule is polar, and include a sketch of the molecule. There are 2 steps to solve this one. There are 26 valence electrons. Web questions » science/math » chemistry ». Web draw the lewis structure for the molecule ch2chch3. To learn about lewis structures, we. How many sigma and pi bonds does it contain? This widget gets the lewis structure of chemical compounds. The number of valence electrons for each atom. Draw an appropriate lewis structure for ch2chch3. 1.2.1 lewis structure of diatomic molecules. There are 2 steps to solve this one. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. The propene lewis structure shows that it has 8 sigma and 1 pi bonds. First, lets find the how many valence electrons chlorate has: Draw the lewis structure for the molecule ch2chch3. Let’s draw and understand this lewis dot structure step by step. A lewis structure is a representation of a molecule or ion that shows. In liquid form, it is volatile and poisonous. To learn about lewis structures, we. This is a dead giveaway that n carries a +1 charge (and no lone pairs). Web expert answer step 1 basic concepts: There are 2 steps to solve this one. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web draw the lewis structure for the molecule ch2chch3. For $\ce{ch2chch3}$ draw an appropriate lewis structure. The number of valence electrons for each atom. Web to draw a lewis structure, it is not necessary to know: Web draw the lewis electron structure of the molecule or polyatomic ion. Draw the molecule by placing atoms on the grid and connecting them with bonds.
List Of Ch2Chch3 Lewis Structure Ideas

How to Draw the Lewis Dot Structure for CH2CHCH3 YouTube

Lewis Structure Of Ch2chch3

Draw the lewis structure for the molecule ch2chch3. how many sigma and
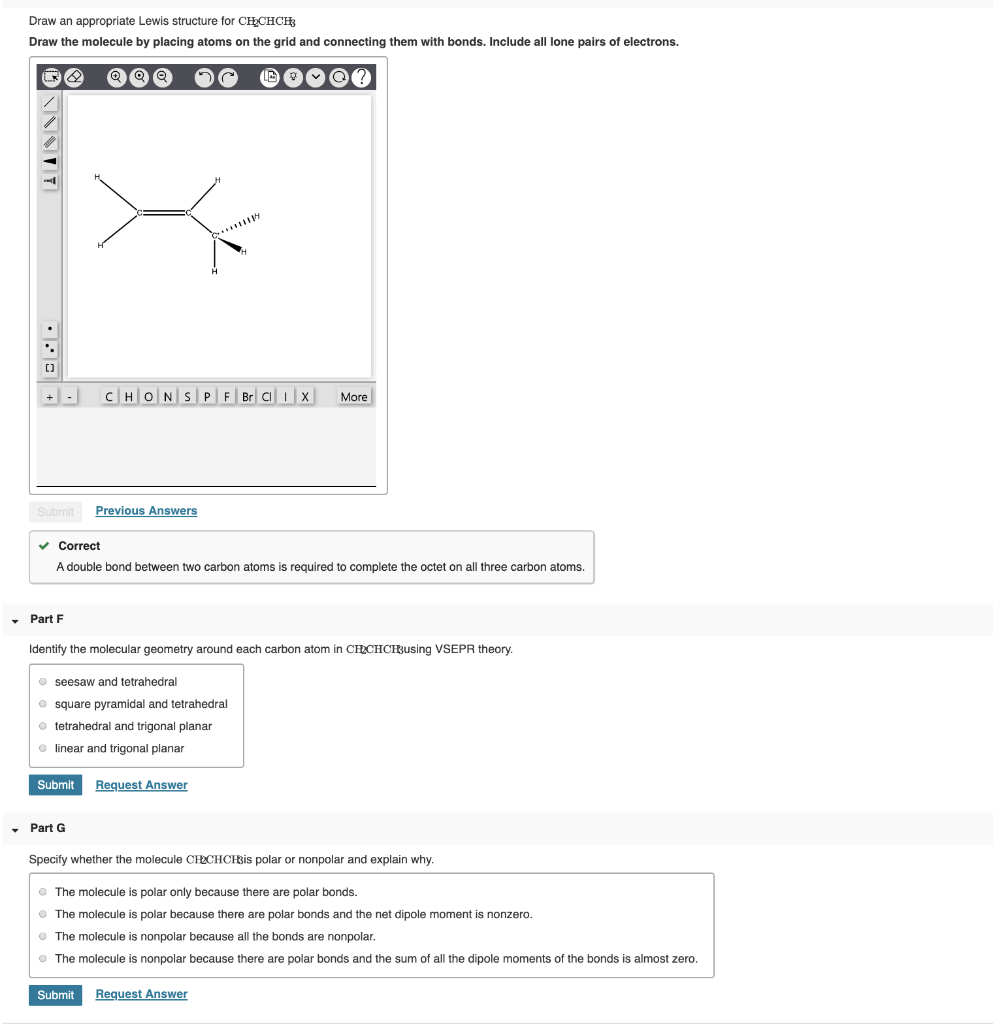
Ch2chch3 Lewis Structure

How to Draw a Lewis Structure

Lewis Structure of Ichloropropane CH3CH2CH2Cl YouTube

Lewis Structure Types
[Solved] Consider the Lewis structure for the molecule CH2CHCH3
(Get Answer) Transcribed image text Lewis structure ill Draw a
Find More Chemistry Widgets In Wolfram|Alpha.
Web In This Example, We Draw The Lewis Structure For The Organic Molecule Ch3Cooh, Acetic Acid, And Evaluate It Using Formal Charge.
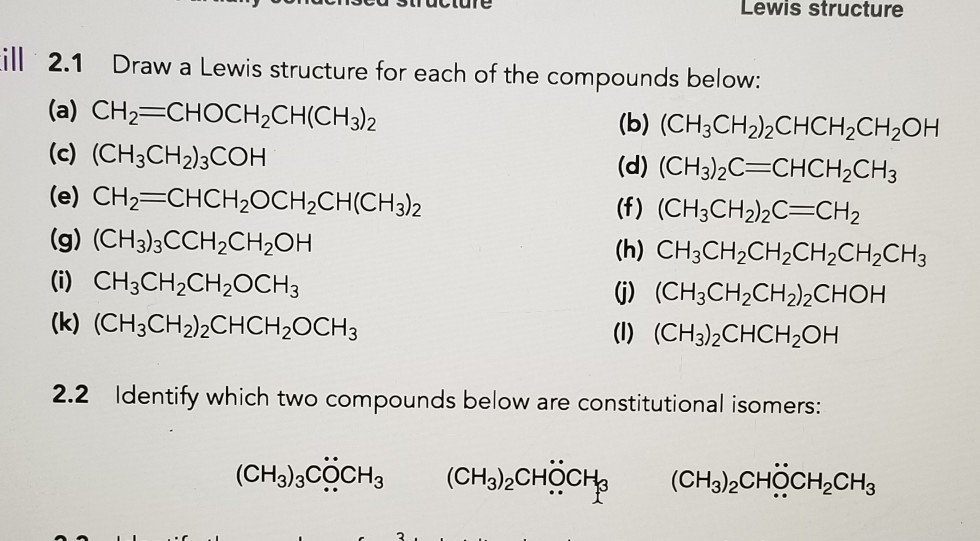
Web 2.1 Draw A Lewis Structure For Each Of The Compounds Below:
Web For Ch2Chch3 Draw An Appropriate Lewis Structure.
Related Post: