Draw An Appropriate Lewis Structure For If5
Draw An Appropriate Lewis Structure For If5 - Web hello, it's time for your daily chemistry dose! Determine the total number of valence (outer shell) electrons. This gives it ax5e1 shape by vsepr, and. Iodine is below period two on the periodic table so it can have. Web a video explanation of how to draw the lewis dot structure for iodine pentafluoride, along with information about the compound including formal charges, pola. For if5, we have a total of 42 valence electrons. Web drawing the lewis structure for if 5. Part a draw an appropriate lewis structure for ifs. Find the total valence electrons in if5 molecule. For the central iodine atom: The first step is to count all the valence electrons of each molecule. Let us follow a few steps. Web drawing the lewis structure for if 5. In the case of if5, the iodine atom has 7. Web 10k views 3 years ago lewis structures. The first step is to count all the valence electrons of each molecule. Web 1) draw a lewis structure for iof5 and calculate the formal charges on each atom. Web chemistry questions and answers. Web drawing the lewis structure for if 5. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Include all lone q (5 ଡ bo identify the geometry of if5 using vsepr theory. This gives it ax5e1 shape by vsepr, and. Determine the total number of valence (outer shell) electrons. For each compound draw an appropriate lewis structure, determine the molecular geometry using. Web science chemistry chemistry questions and answers draw the lewis dot structure of the molecule if5 and determine the electron and molecular geometries around the i atom. Part a draw an appropriate lewis structure for ifs. Valance electron determination considering the if5 lewis structure, both iodine and fluorine atom contain 7 valence electrons. Figure out how many electrons the molecule. Lewis structure of if5 for counting valence electrons around the terminal fluorine atoms. Web 1) draw a lewis structure for iof5 and calculate the formal charges on each atom. Which has the larger bond angle? Web drawing the lewis structure for if 5. Here, the given molecule is if5 (iodine pentafluoride). Remember that iodine (i) can hold more than eight valence electrons. Web 1) draw a lewis structure for iof5 and calculate the formal charges on each atom. Which has the larger bond angle? For each compound draw an appropriate lewis structure, determine the molecular geometry using vsepr theory, determine whether the molecule is polar and identify the hybridization of all. In the case of if5, the iodine atom has 7. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Web 1) draw a lewis structure for iof5 and calculate the formal charges on each. Draw the molecule by placing atoms on the grid and connecting them with bonds. Put one electron pair in each bond 4. This is the if5 lewis structure. In the case of if5, the iodine atom has 7. Find more chemistry widgets in wolfram|alpha. Iodine is the least electronegative. Which has the larger bond angle? Web steps of drawing if5 lewis structure step 1: 2) draw a lewis structure for if5 and calculate the formal charges on each atom. This gives it ax5e1 shape by vsepr, and. For the central iodine atom: Put one electron pair in each bond 4. Fill outer atoms with electrons 5. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. The number of lone pairs = the number. Web steps of drawing if5 lewis structure step 1: 3) describe the bonding in the following molecules bonding molecule. Determine the total number of valence (outer shell) electrons. See the big list of lewis structures. Web drawing the lewis structure for if 5. This is the if5 lewis structure. Calculate the total number of valence electrons. For the central iodine atom: Web chemistry questions and answers. The central iodine atom a. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Here, the given molecule is if5 (iodine pentafluoride). For if5, we have a total of 42 valence electrons. Put one electron pair in each bond 4. Valance electron determination considering the if5 lewis structure, both iodine and fluorine atom contain 7 valence electrons. Web science chemistry chemistry questions and answers draw (on paper) a lewis structure for if_5 and answer the following questions based on your drawing.
IF5 Lewis, VSEPR and Box diagram for hybridiaztion YouTube
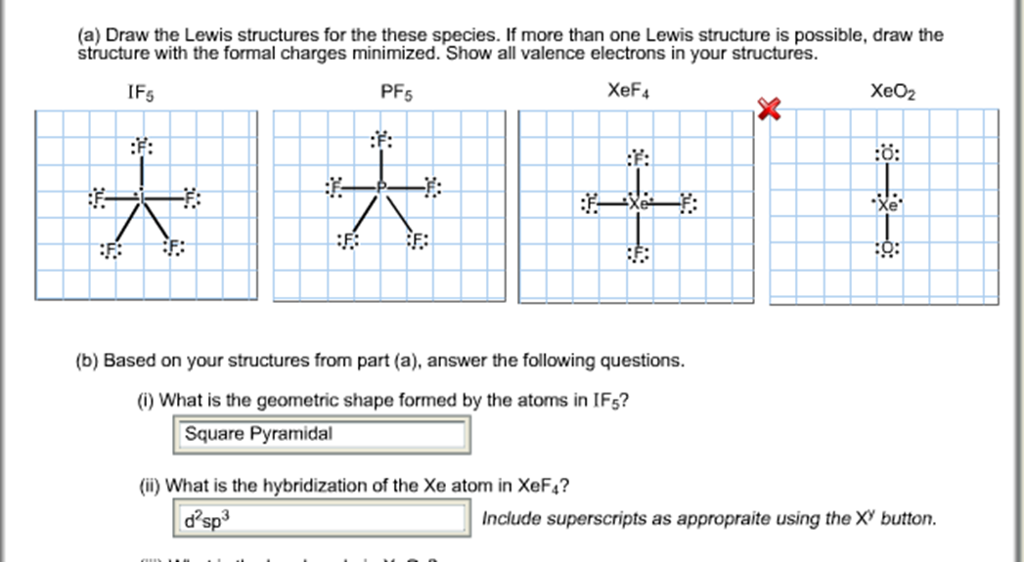
If5 Lewis Structure
If5 Lewis Structure

IF5 Molecular Geometry Science Education and Tutorials
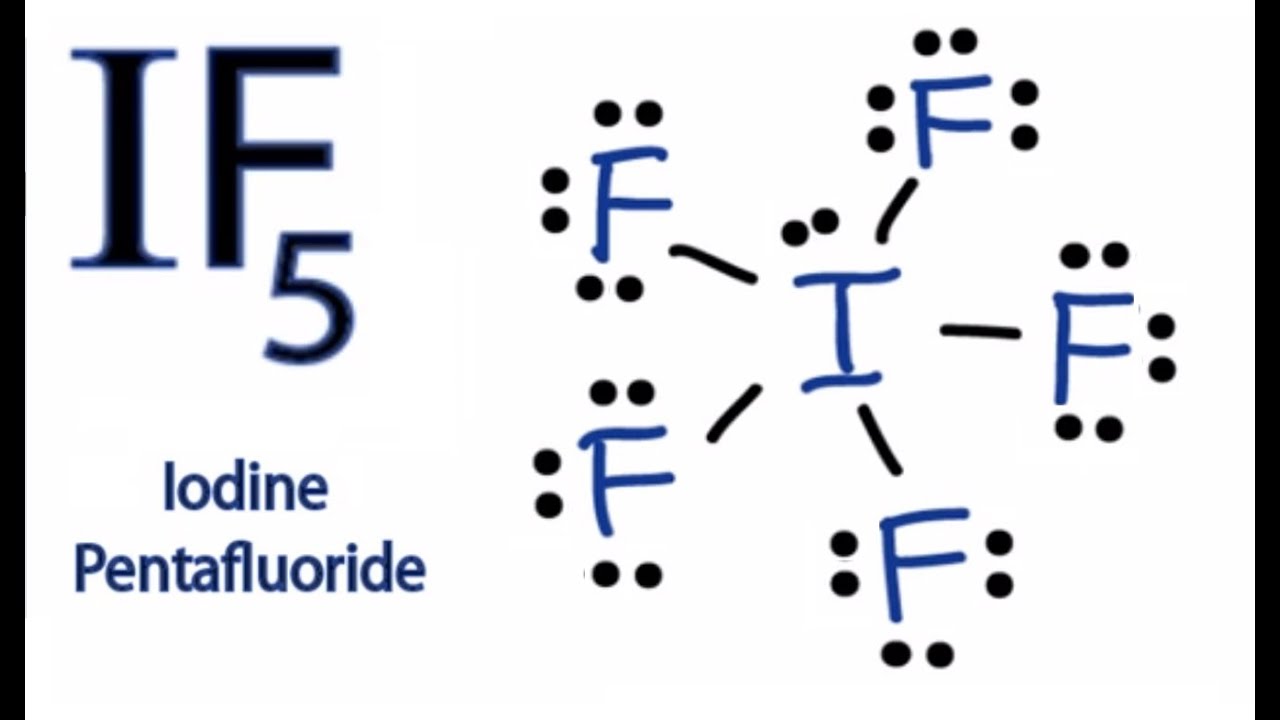
IF5 Lewis Structure How to Draw the Lewis Structure for IF5 YouTube
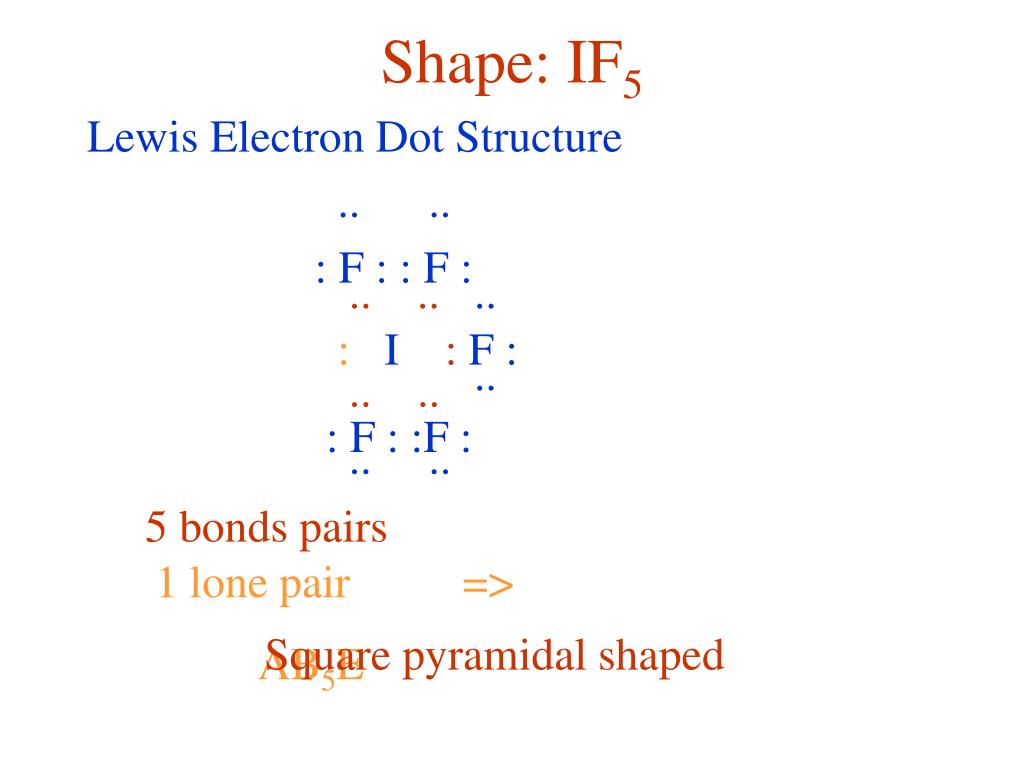
If5 Lewis Structure

IF5 Molecular Geometry, Bond Angles and Electron Geometry YouTube

If5 Lewis Structure

If5 Lewis Structure
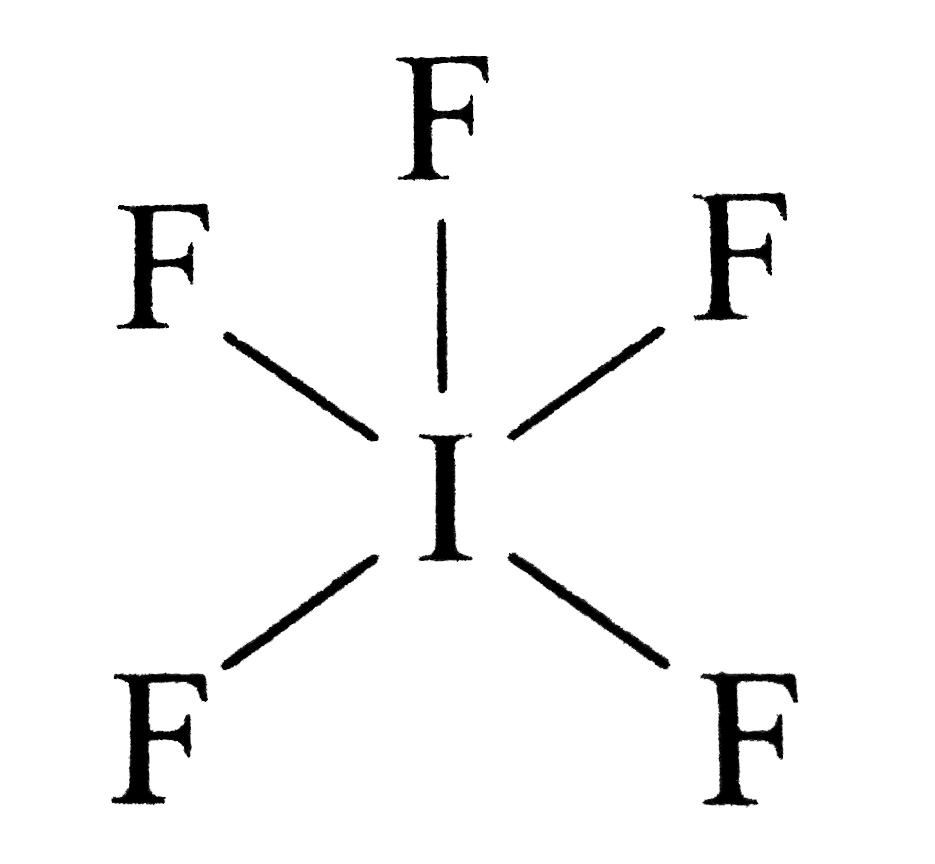
Draw the Lewis structure of iodine pentafluoride, IF5.
2) Draw A Lewis Structure For If5 And Calculate The Formal Charges On Each Atom.
The Number Of Lone Pairs = The Number Of Single Bonds = The Number Of Double Bonds = 2.
Iodine Pentafluoride (If5) Has 5 Fluorine Atoms Bonded To A Central Iodine, And That Iodine Has One Lone Pair On It.
Web 1) Draw A Lewis Structure For Iof5 And Calculate The Formal Charges On Each Atom.
Related Post: