Draw And Label A Water Molecule
Draw And Label A Water Molecule - Water, carbon dioxide and energy. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Generate the γ γ 's. Web students will be able to explain, on the molecular level, what makes water a polar molecule. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. At the molecular scale, this “surface” may be a single crystal platform, a step, a biological film, or a macromolecule. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Make sure to include the terms, 'solute', 'solvent' and 'soluble'. Web study with quizlet and memorize flashcards containing terms like write the chemical equation for water, draw a stick model of a water molecule, sketch a space filling model of a water molecule, show polarity by labeling positive and negative charged regions of the molecule. All of the electron pairs—shared and unshared—repel each other. It can be obtained by ingestion of liquids, food or by the combustion of food, as this reaction releases: Learn vocabulary, terms, and more with flashcards, games, and other study tools. Web draw a model of a water molecule. Web 0:00 / 0:56 3.1.4 draw and label a diagram showing the. Web learn about molecules and the water molecule definition. Make sure to include the terms, 'solute', 'solvent' and 'soluble'. About 70% of the human body’s weight is composed of this molecule. Web to understand water at the molecular scale, we have to study the interaction between water and the surface in detail. Web because the water molecule has an h. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Complete the labels showing the locations of the hydrogen atoms, the oxygen atom, and the regions of positive and negative charge. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. So, water molecules. Web to understand water at the molecular scale, we have to study the interaction between water and the surface in detail. At the molecular scale, this “surface” may be a single crystal platform, a step, a biological film, or a macromolecule. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Web start. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Colorless, odorless and tasteless, water is one of the most important elements for all known life forms. Web molecular structure of water: Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web. Label the atoms that make up the molecule, as well as their partial charges. Oxygen is highly electronegative, which creates a partial negative charge on one end of the molecule, and a partial positive charge on the other. Colorless, odorless and tasteless, water is one of the most important elements for all known life forms. So, water molecules are able. Web to understand water at the molecular scale, we have to study the interaction between water and the surface in detail. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Students will also be able to show in a drawing. Web study with quizlet and memorize flashcards containing terms like write the chemical equation for water, draw a stick model of a water molecule, sketch a space filling model of a water molecule, show polarity by labeling positive and negative charged regions of the molecule. Colorless, odorless and tasteless, water is one of the most important elements for all known. Find the point group of the molecule and assign cartesian coordinates so that z is the principal axis. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Label with the element symbol, charge, and place the electrons. When drawing the structure of an ion, be sure to add/subtract electrons to account for. Explain what a solution is. Web molecular structure of water: Water, carbon dioxide and energy. Identify and count the pendant atoms' valence orbitals. Label the bond between hydrogen and oxygen as polar covalent bond. Explain what a solution is. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Learn about the water molecule structure, its properties, and what makes a molecule of water polarized. Web to understand water at the molecular scale, we have to study the interaction between water and the surface in detail. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Label the bond between hydrogen and oxygen as polar covalent bond. Label the atoms that make up the molecule, as well as their partial charges. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web learn about molecules and the water molecule definition. Water also attracts or is attracted to other polar molecules and ions. Web answer 19 people found it helpful billiejean05 report flag outlined answer: Web draw and label a ball and stick presentation of a water molecule. Complete the labels showing the locations of the hydrogen atoms, the oxygen atom, and the regions of positive and negative charge.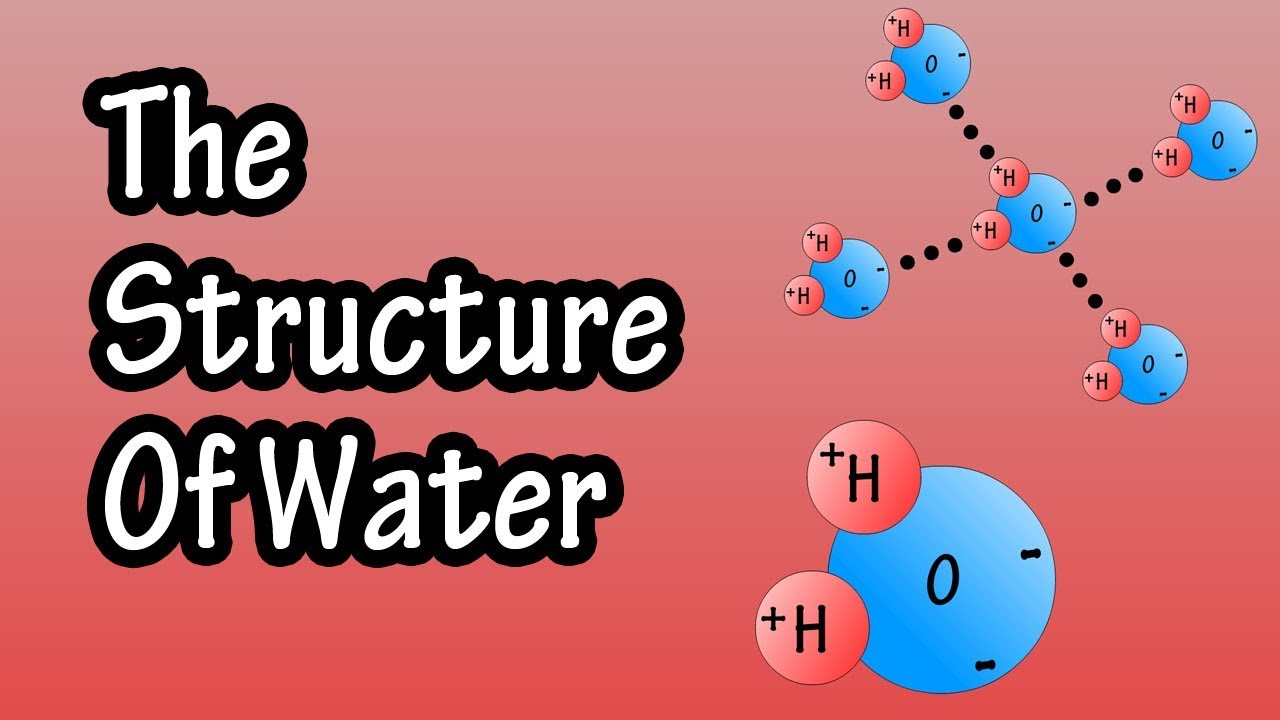
Structure Of Water Molecule Chemistry Of Water Properties Of Water

Diagram Of Water Molecule

Diagram Of Water Molecule
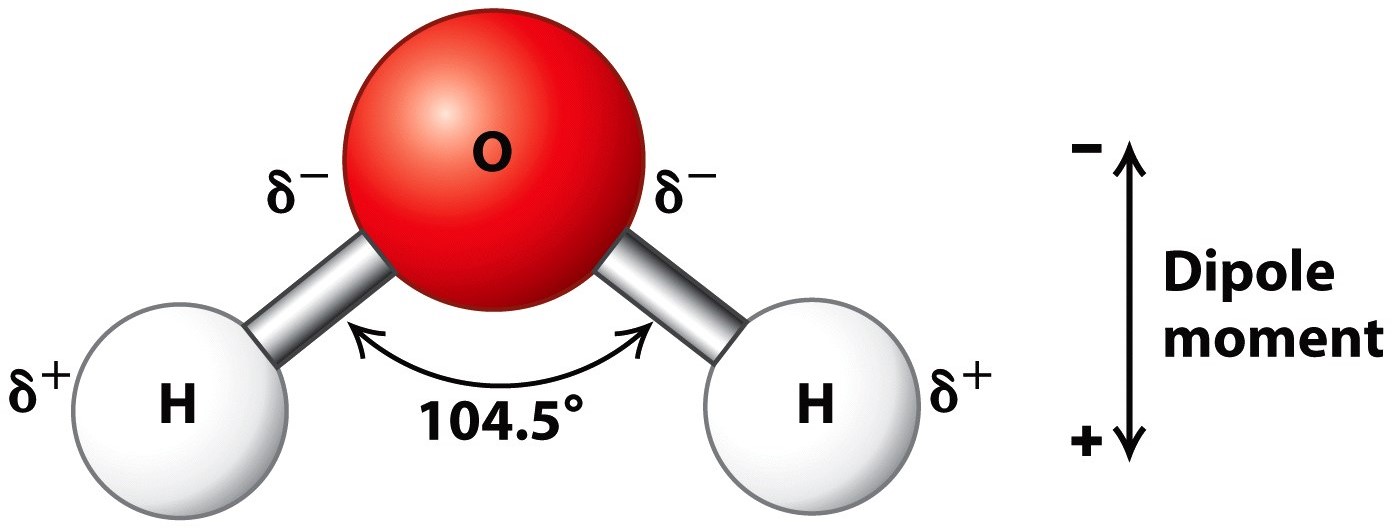
Describe the Structure of a Water Molecule
Science online The importance of the water and its structure

Types of Atoms Science at Your Doorstep

Draw a neat well labelled diagram of information of water molecule
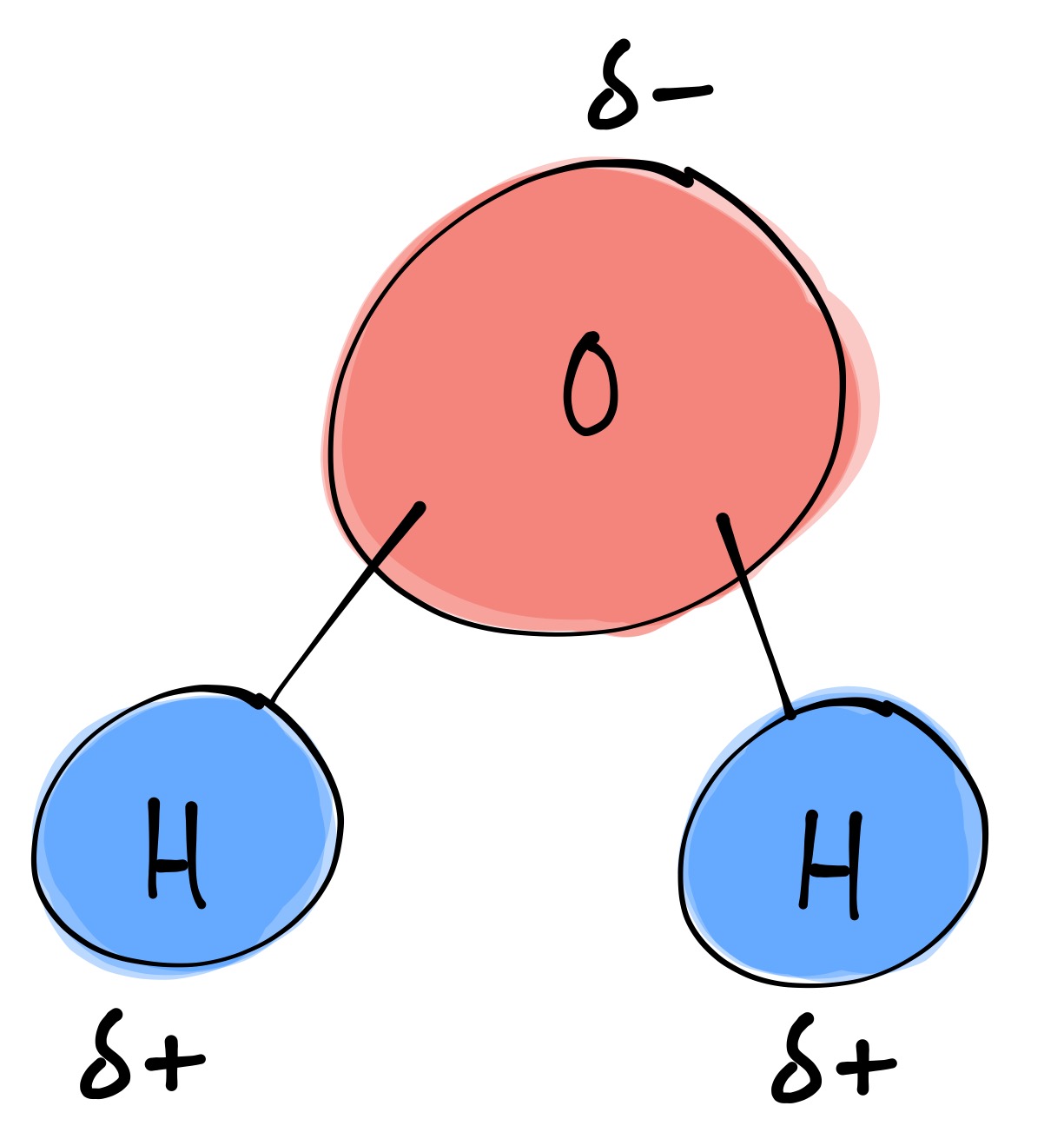
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule
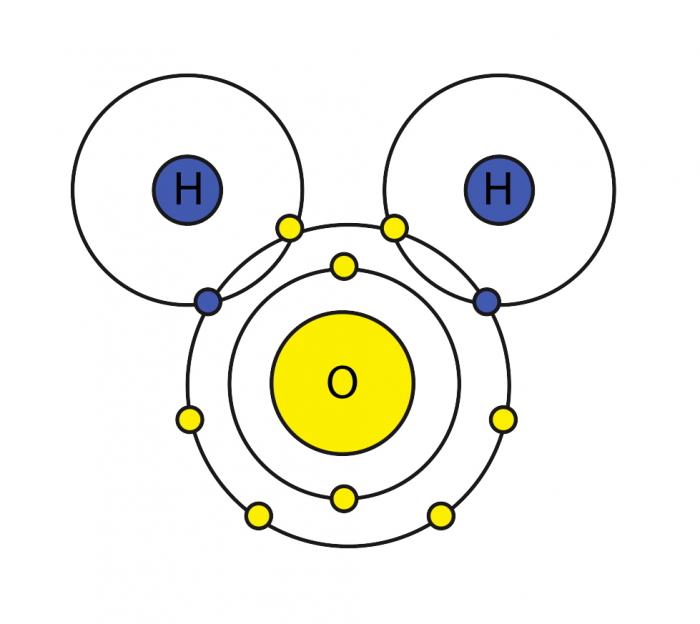
The Configuration of the Water Molecule EARTH 111 Water Science and
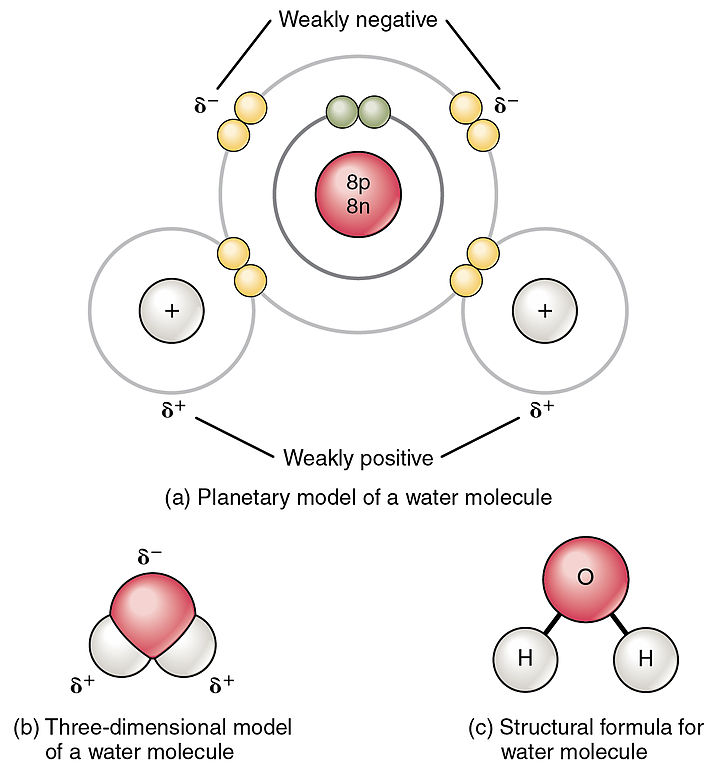
Water Principles of Biology
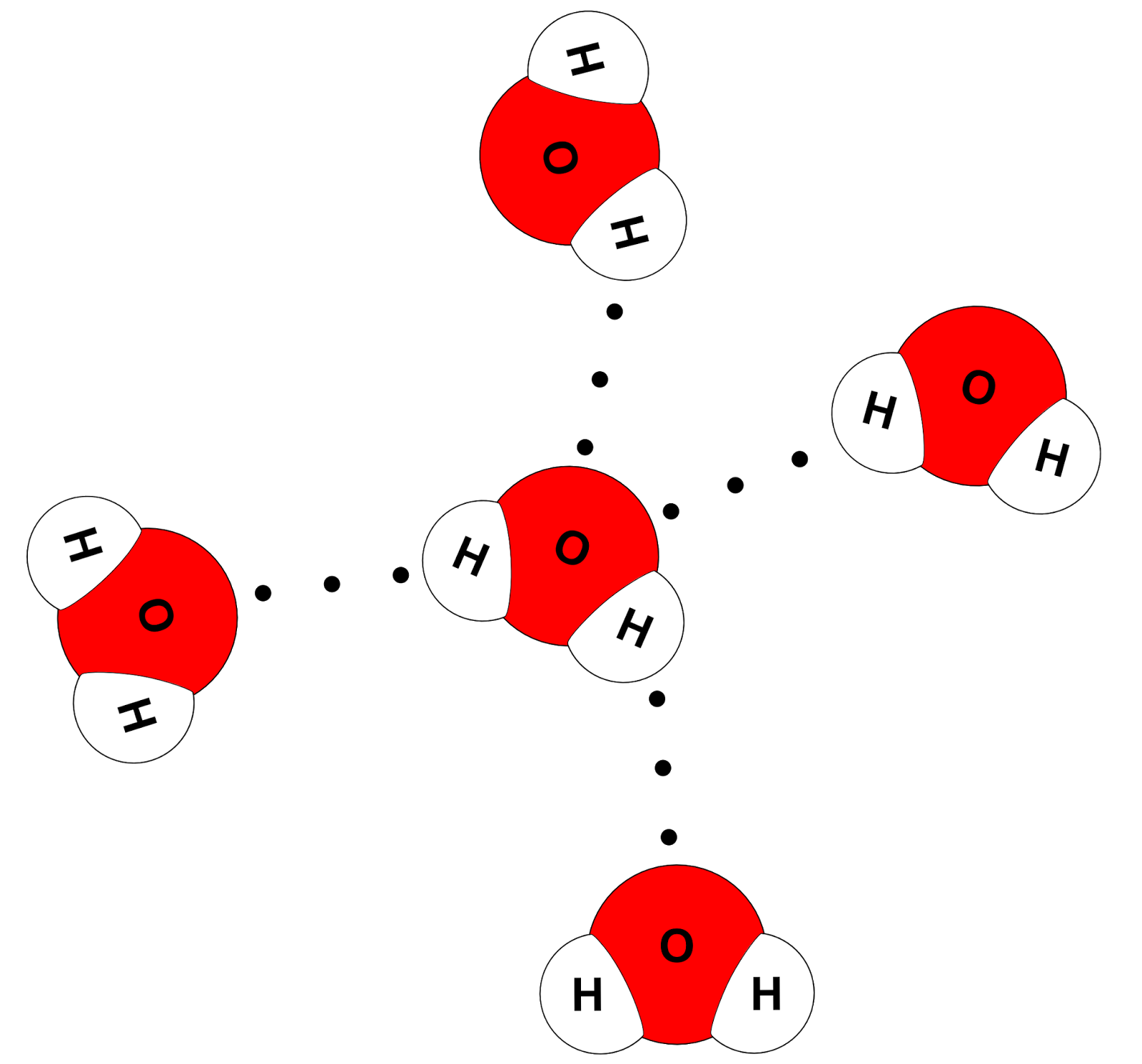
Web As A Result Of Water’s Polarity, Each Water Molecule Attracts Other Water Molecules Because Of The Opposite Charges Between Water Molecules, Forming Hydrogen Bonds.
The Covalent Bonds Are The Bonds That Connect The Parts Of The Water Molecule Together And The Hydrogen Bonds Connect The Individual Molecules Together.
A Solution Is A Liquid Mixture Of A Solvent (Substance That Dissolves), And A Solute (Substance Being Dissolved By Solvent).
Explain What Is Meant By Hydrogen Bonding And The Molecular Structural Features That Bring It About.
Related Post: