Draw And Label Water Molecule
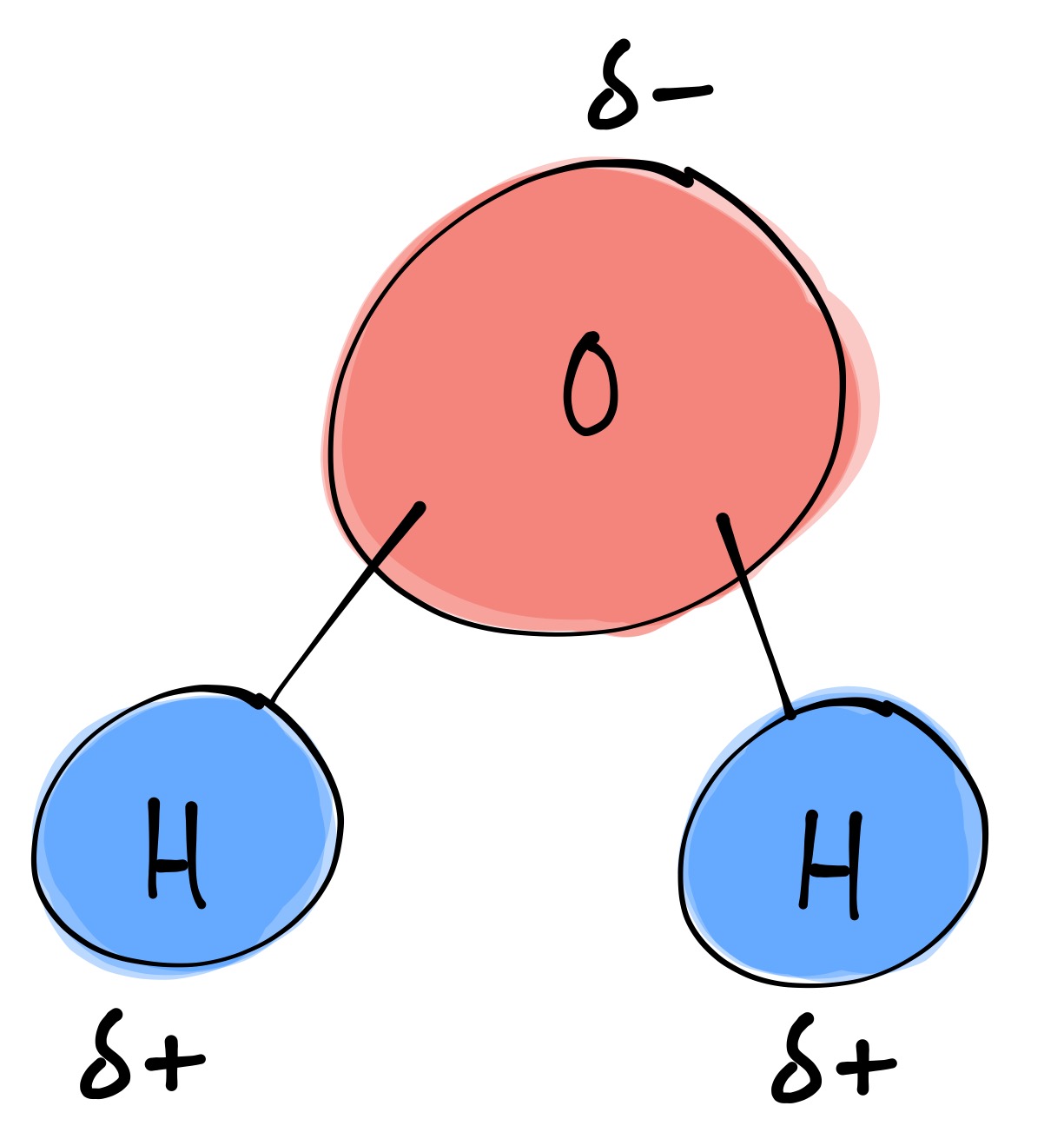
Draw And Label Water Molecule - The molecular geometry and the shape of the water molecule are bent due to the repulsion forces of lone pairs. Without it, life as we know it simply would not exist. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web this drawing highlights two h 2 o molecules, one at the surface, and the other in the bulk of the liquid. Web each water molecule links to four others creating a tetrahedral arrangement, however they are able to move freely and slide past each other, while ice forms a solid, larger hexagonal structure. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Web draw a model of a water molecule. Use circles to designate atoms and lines for bonds, then indicate the chemical symbol for each atom. Water molecule definition molecular polarity the atomic structure of water lesson summary frequently asked. A solution is a liquid mixture of a solvent (substance that dissolves), and a solute (substance being dissolved by solvent). Explain what a solution is. Label the bond between hydrogen and oxygen as polar covalent bond. Without it, life as we know it simply would not. We will walk through the steps below to construct the molecular orbital diagram of water. The covalent bonds are the bonds that connect the parts of the water molecule together and the hydrogen bonds connect the individual molecules together. Web each water molecule links to four others creating a tetrahedral arrangement, however they are able to move freely and slide. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Make sure to include the terms, 'solute', 'solvent' and 'soluble'. Gas state (steam) as water boils, its hydrogen bonds are broken. Web the configuration of the water molecule. Web draw a water molecule with charges. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web because the water molecule has an h — o — h bond angle of 105°, the molecule as a whole is polar. Without it, life as we know it simply would not exist. Web there are two lone pairs. The covalent bonds are the bonds that connect the parts of the water molecule together and the hydrogen bonds connect the individual molecules together. Make sure to include the terms, 'solute', 'solvent' and 'soluble'. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. These properties allow cells to regulate their internal temperature,. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Web study with quizlet and memorize flashcards containing terms like write the chemical equation for water, draw a stick model of a water molecule, sketch a space filling model of a water molecule, show polarity by labeling positive and negative charged regions of. The diagram below depicts a water molecule. Try the fastest way to create flashcards Without it, life as we know it simply would not exist. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. There are two lone pairs of electrons on each oxygen atom (represented by. Web draw and label a ball and stick presentation of a water molecule. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. There. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. Steam particles move very far apart and fast, so barely any hydrogen bonds have the. Gas state (steam) as water boils, its hydrogen bonds are broken. The surface molecule is attracted to its neighbors below and to either side, but. Label with the element symbol, charge, and place the electrons. The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the processes of life. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. A molecule of. Water is a polar molecule, as greater electron density is found around the more electronegative oxygen atom. Label with the element symbol, charge, and place the electrons. Without it, life as we know it simply would not exist. Label the bond between hydrogen and oxygen as polar covalent bond. Web study with quizlet and memorize flashcards containing terms like write the chemical equation for water, draw a stick model of a water molecule, sketch a space filling model of a water molecule, show polarity by labeling positive and negative charged regions of the molecule. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. Web draw a model of a water molecule. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Make sure to include the terms, 'solute', 'solvent' and 'soluble'. Web the configuration of the water molecule. There are two lone pairs of electrons on each oxygen atom (represented by. The diagram below depicts a water molecule. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. The surface molecule is attracted to its neighbors below and to either side, but there are no attractions pointing in. Web draw a water molecule with charges.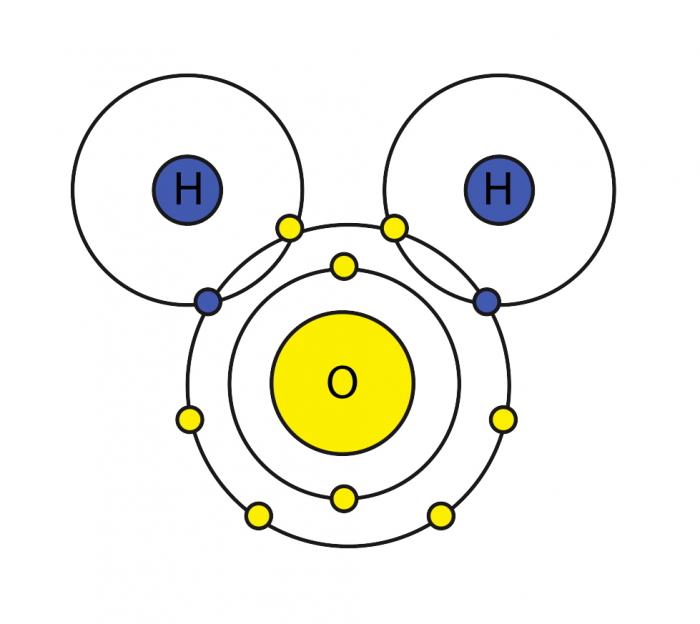
The Configuration of the Water Molecule EARTH 111 Water Science and
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule
Structure Of Water Molecule Chemistry Of Water Properties Of Water

Draw a neat well labelled diagram of information of water molecule

Types of Atoms Science at Your Doorstep
Science online The importance of the water and its structure

Diagram Of Water Molecule

Diagram Of Water Molecule
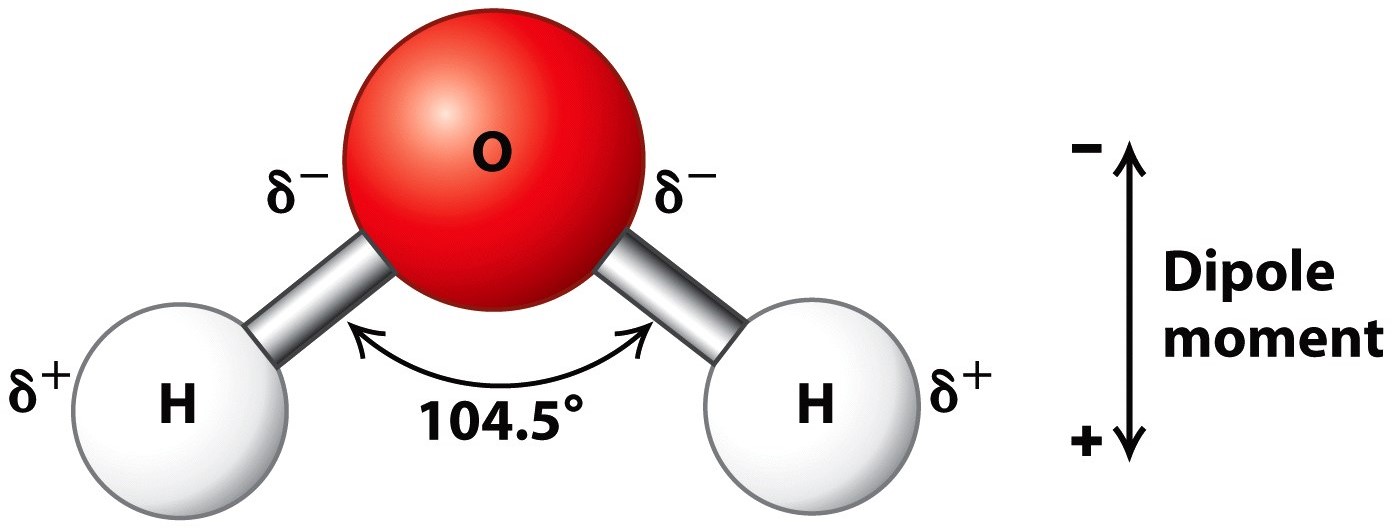
Describe the Structure of a Water Molecule
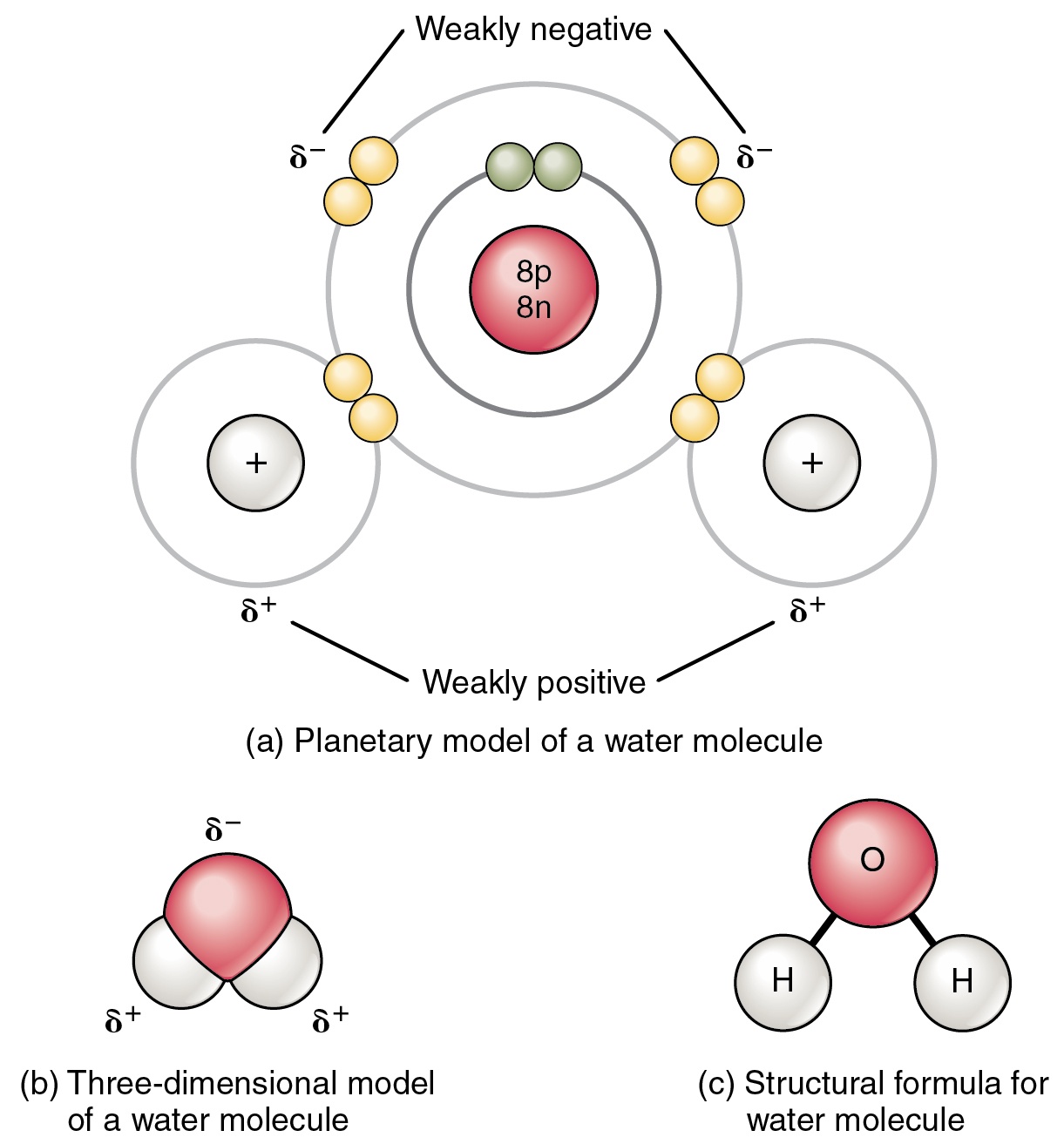
Water — Molecular Structure & Bonding Expii
The Molecular Geometry And The Shape Of The Water Molecule Are Bent Due To The Repulsion Forces Of Lone Pairs.
Learn Vocabulary, Terms, And More With Flashcards, Games, And Other Study Tools.
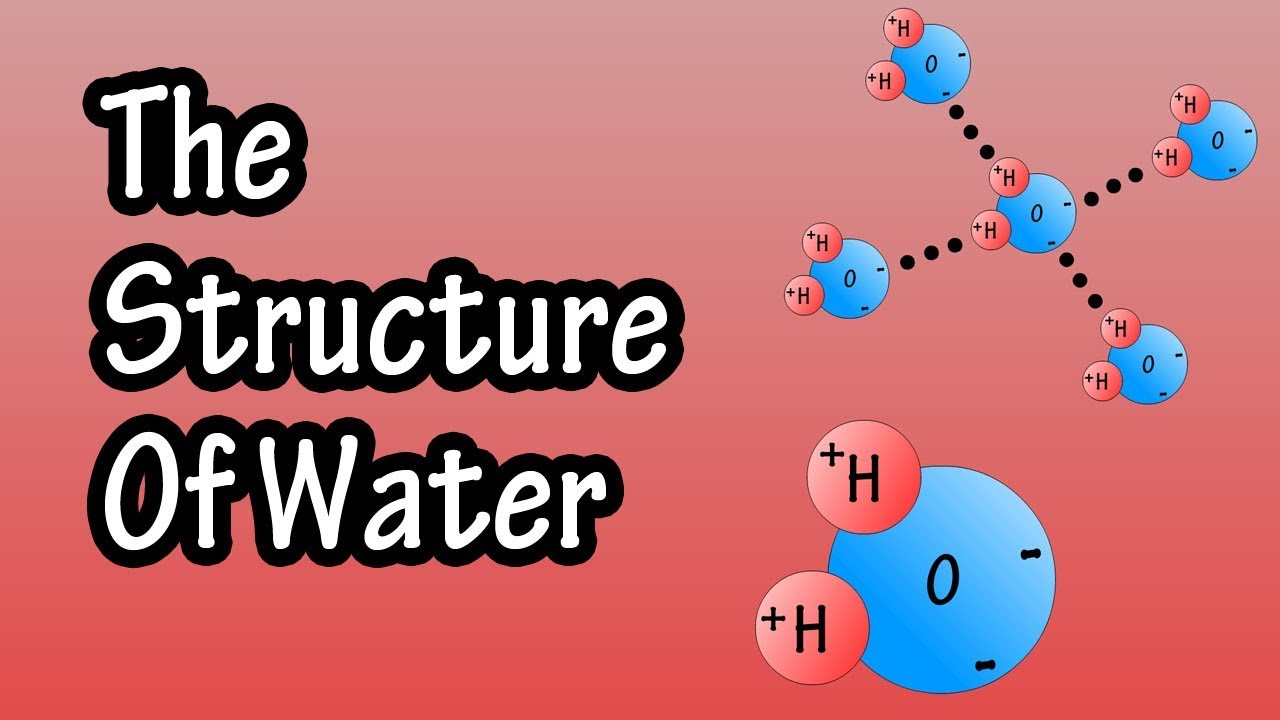
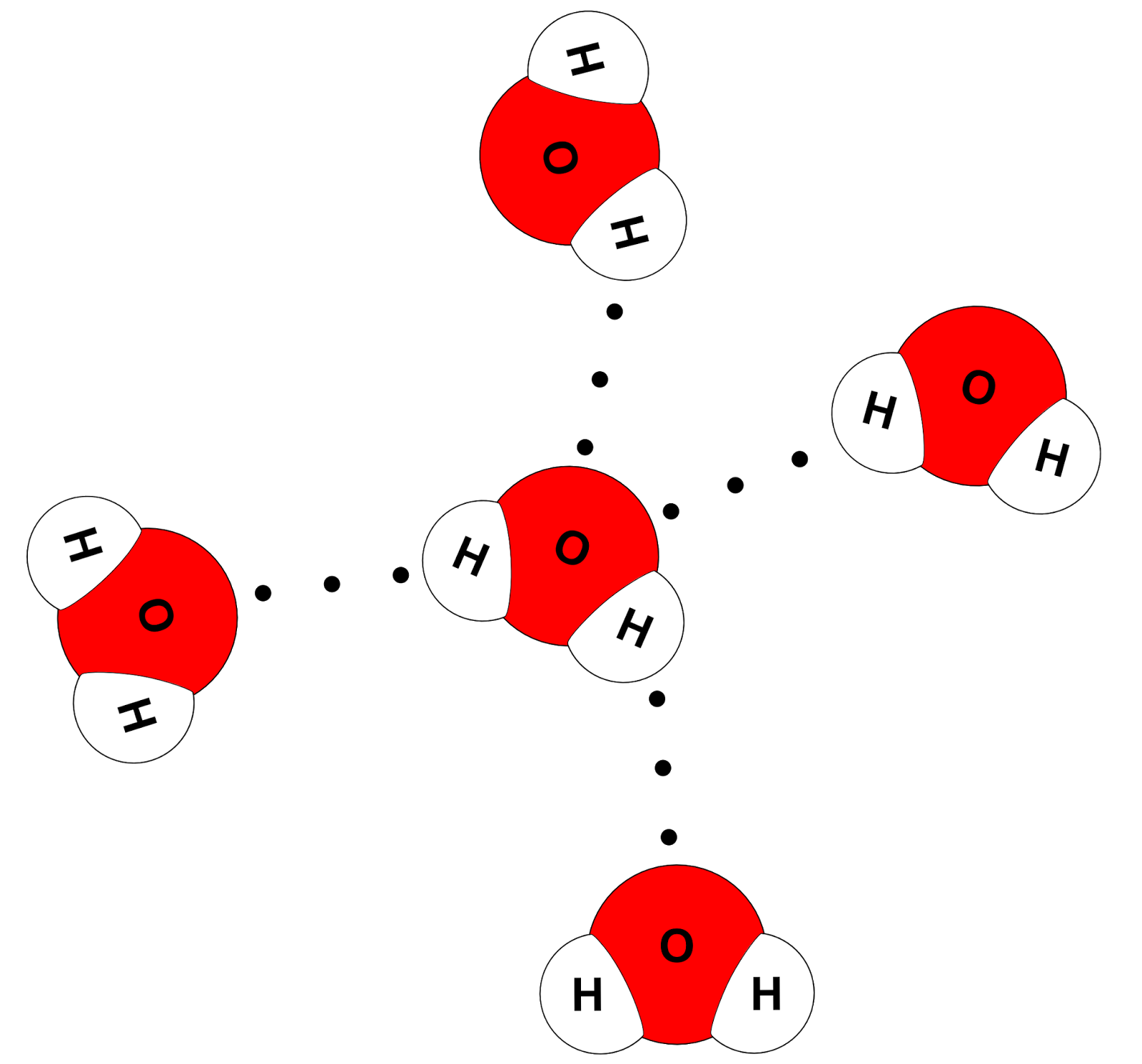
Web Hydrogen Bonds Between Water Molecules Give Water Its High Boiling Point, High Heat Capacity, And Surface Tension.
Web This Drawing Highlights Two H 2 O Molecules, One At The Surface, And The Other In The Bulk Of The Liquid.
Related Post: