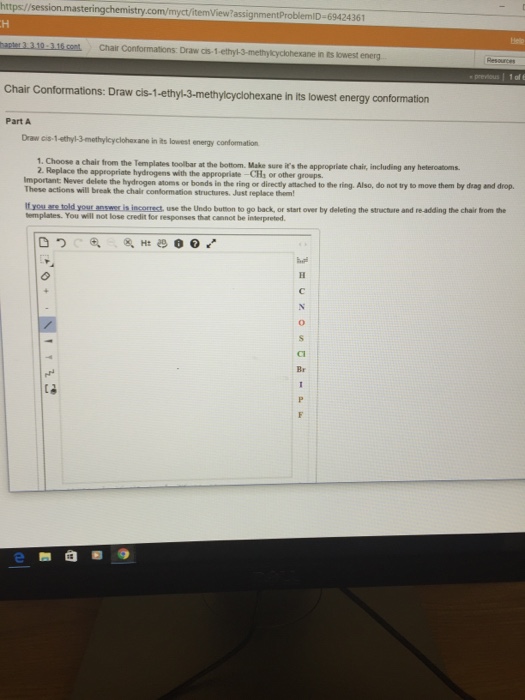
Draw Cis-1-Ethyl-3-Methylcyclohexane In Its Lowest Energy Conformation
Draw Cis-1-Ethyl-3-Methylcyclohexane In Its Lowest Energy Conformation - You should get something like this: Ensure both groups are on the same side of the ring (cis configuration). Web 17) the energy difference between the axial and equatorial conformers of methylcyclohexane is: Choose a chair from the templates toolbar at the bottom. Make sure it's the appropriate chair, including any heteroatoms. Web the single bond is active default gh: Copy sheet of paper on top of another sheet. Make sure it's the appropriate chair, including any. Make sure it?s the appropriate chair, including any heteroatoms. Use this link for bookmarking this species for future reference. Use this link for bookmarking this species for future reference. Trans 1 is the first compound. You should get something like this: The groups must both be on the same side of the ring (both up or both down). Choose a chair from the templates toolbar at the bottom. Make sure it's the appropriate chair, including any heteroatoms. Never delete the hydrogen atoms or bonds in the ring or directly attached. Ensure both groups are on the same side of the ring (cis configuration). Next, place the ethyl group (c2h5) on carbon 1 and the methyl group (ch3) on carbon 3. This structure is also available as a 2d. Choose a chair from the templates toolbar at the bottom. Choose a chair from the templates toolbar at the bottom. Choose a chair from the templates toolbar at the bottom. The metal group will present in the axil portion in the second confirm and the other metal group will present in Choose a chair from the templates toolbar at the. Choose a chair from the templates toolbar at the bottom. Trans 1 is the first compound. Make sure it?s the appropriate chair, including any heteroatoms. Ensure both groups are on the same side of the ring (cis configuration). First draw the two chair forms, then add the ethyl and methyl groups to carbons 1 and 3. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Ensure both groups are on the same side of the ring (cis configuration). Choose a chair from the templates toolbar at the bottom. First draw the two chair forms, then add the ethyl. We have to find out the flow energy child conformer of 2 compounds, given in the question. Choose a chair from the templates toolbar at the bottom. Web 17) the energy difference between the axial and equatorial conformers of methylcyclohexane is: First draw the two chair forms, then add the ethyl and methyl groups to carbons 1 and 3. Never. Choose chair from the templates toolbar at the bottom_ make sure its the appropriate chair; The metal group will present in the axil portion in the second confirm and the other metal group will present in Make sure it's the appropriate chair, including any. This structure is also available as a 2d mol file or as a computed 3d sd. Make sure it?s the appropriate chair, including any heteroatoms. Ensure both groups are on the same side of the ring (cis configuration). Make sure it's the appropriate chair, including any heteroatoms. Build a model of cyclohexanol in the most stable chair form. Next, place the ethyl group (c2h5) on carbon 1 and the methyl group (ch3) on carbon 3. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Make sure it's the appropriate chair, including any heteroatoms. Make sure it's the appropriate chair, including any heteroatoms. Build a model of cyclohexanol in the most stable chair form. Choose a chair from. Choose chair from the templates toolbar at the bottom_ make sure its the appropriate chair; Never delete the hydrogen atoms or bonds in the ring or directly attached. Make sure it?s the appropriate chair, including any heteroatoms. Next, place the ethyl group (c2h5) on carbon 1 and the methyl group (ch3) on carbon 3. Make sure it's the appropriate chair,. Ensure both groups are on the same side of the ring (cis configuration). Make sure it's the appropriate chair, including any. Use this link for bookmarking this species for future reference. Choose a chair from the templates toolbar at the bottom. First draw the two chair forms, then add the ethyl and methyl groups to carbons 1 and 3. Web the single bond is active default gh: We have to find out the flow energy child conformer of 2 compounds, given in the question. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. The groups must both be on the same side of the ring (both up or both down). Choose a chair from the templates toolbar at the bottom. Draw the most stable (preferred) conformation for each molecule listed below in (i) and (ii). You should get something like this: Including any replace the appropriate hydrogens with the appropriate chz or other groups_ important: Never delete the hydrogen atoms or bonds in the ring or directly attached. Next, place the ethyl group (c2h5) on carbon 1 and the methyl group (ch3) on carbon 3. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.draw structure of 1 ethyl 3 methyl cyclo hexane and structure of i
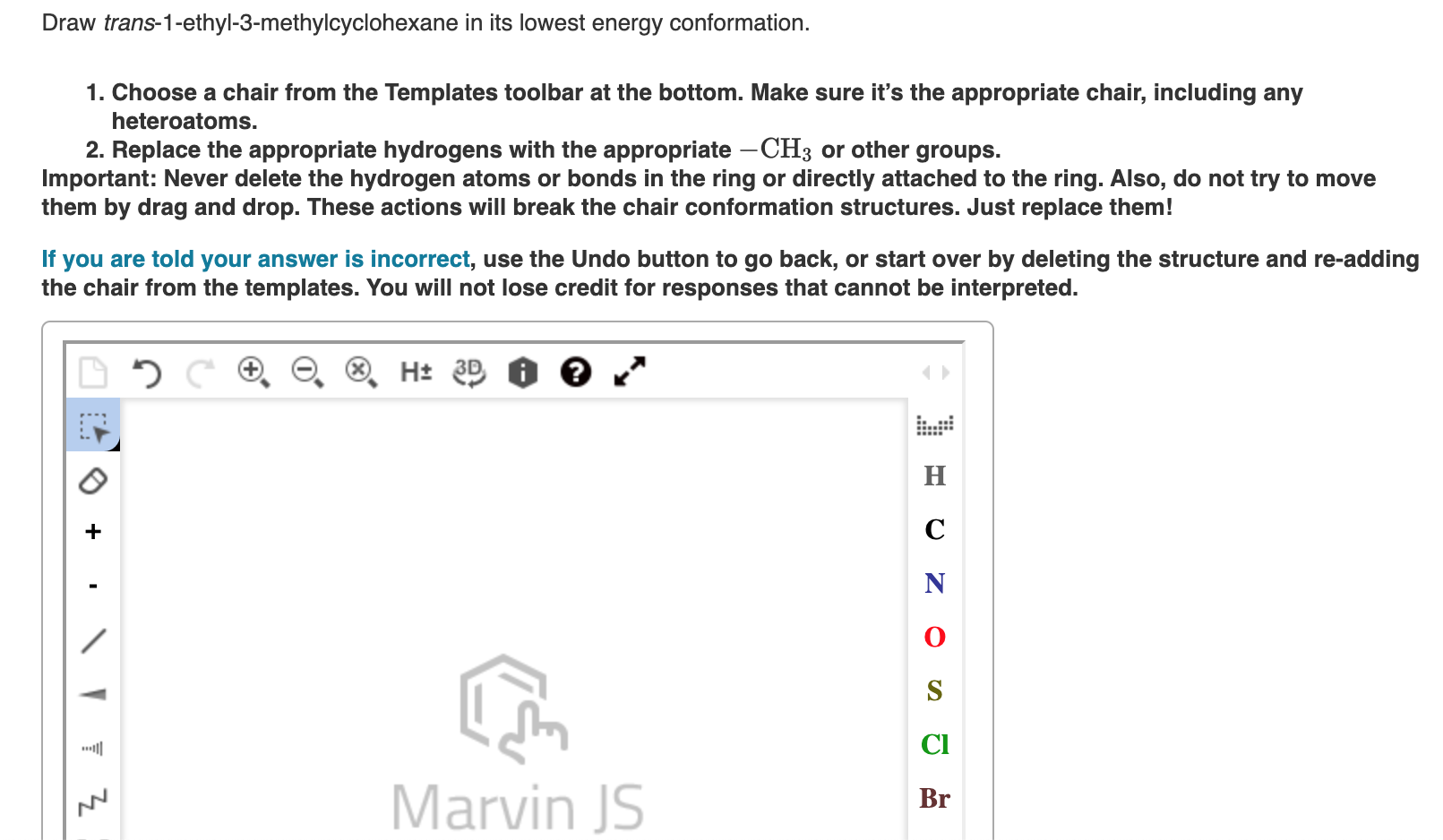
Solved Draw trans1ethyl3methylcyclohexane in its lowest

Compare the stabilities of the following two compounds A cis1Ethy
20. Draw the most stable conformation of (cis)1ethy… SolvedLib
Solved Part A cis1ethyl3methylcyclohexane Drag the

1Ethyl3methylcyclohexane (cis and trans mixture) 98.0 , TCI
Solved Draw cis1ethyl3methylcyclohexane in its lowest
how many stereoisomers are there for 1 ethyl 3 methylcyclohexane?

OneClass Draw the most stable conformer of cis1ethyl3
cis1Ethyl3methylcyclohexane
Build A Model Of Cyclohexanol In The Most Stable Chair Form.
Choose A Chair From The Templates Toolbar At The Bottom.
Choose A Chair From The Templates Toolbar At The Bottom.
Web 17) The Energy Difference Between The Axial And Equatorial Conformers Of Methylcyclohexane Is:
Related Post: