Draw Lewis Structure Practice
Draw Lewis Structure Practice - (diacetylene may be a little tricky!) answer. Lewis diagram of formaldehyde (ch₂o) worked example: Web in these practice problems, we will work on determining the lewis structures of molecules and ions. Web lewis structures practice worksheet draw the lewis structures for each of the following molecules. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Draw the best lewis dot structures for each of the following species. This chemistry video provides a basic introduction into how to draw lewis structures of common molecules such as cl2, o2, of2, ch4, nh3, h2o, c2h2, and n2h4. Web practice drawing lewis structures with answers and explanation. Find the total valence electrons. Web answers to chapter 1 practice questions. Write the lewis structures for each of these molecules. Step 3) add electrons to. Web draw the best lewis dot structure for each of the following species. A) bef 2 b) bcl 3 c) ccl 4 d) pbr 5 e) si 6. 3m 7 comments mark as completed was this helpful? Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web draw lewis structures for the following molecular formulas: Web draw the best lewis dot structure for each of the following species. Practice drawing these lewis structures and don't worry we will go over all the answers step. C 2 h 4 (one double bond), c 4 h 6 (two double bonds), and c 4 h 6 (one triple bond). Web 1 concept how to use organic chemistry to make lewis structures easier. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Determine the shape. You should consult the lewis structure rules and. A lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms. Select your preferences below and click 'start' to give it a try! 4m 7 comments mark as completed was this helpful? Drawing lewis structures for bf 3,. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Web things to remember 1. You should consult the lewis structure rules and. 3m 7 comments mark as completed was this helpful? Draw the lewis structure, then id shape and polarity. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Web lewis structures practice worksheet draw the lewis structures for each of the following molecules. Web things to remember 1. Find the total valence electrons. Web draw the lewis dot structure for each of the following polyatomic ions: Lewis diagram of xenon difluoride (xef₂) lewis diagrams. (diacetylene may be a little tricky!) answer. 2 example drawing the lewis structure for n2h4. Web lewis structures practice worksheet draw the lewis structures for each of the following molecules. Web practice with drawing lewis structures introduction to lewis structures the only thing smaller than atoms is their subatomic particles; 2 example drawing the lewis structure for n2h4. Draw the best lewis dot structures for each of the following species. Find the total number of valence electrons in this step, add up the total number of valence electrons from all the atoms in the molecule. Select your preferences below and click 'start' to give it a try! Find the total. Web lewis structures practice worksheet draw the lewis structures for each of the following molecules. Web practice with drawing lewis structures introduction to lewis structures the only thing smaller than atoms is their subatomic particles; Lewis diagram of xenon difluoride (xef₂) lewis diagrams. In short, remember these steps for determining the lewis structure: The following procedure can be used to. Lewis diagram of xenon difluoride (xef₂) lewis diagrams. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Find the number of electrons needed to make the atoms happy an atom is considered happy when its outer electron shell is filled. Find the total number of valence electrons in this step, add up the total number of valence. Web practice drawing lewis structures with answers and explanation. You should consult the lewis structure rules and. To practice predicting molecular shapes (using vsepr theory) and molecular polarity Select your preferences below and click 'start' to give it a try! Lewis diagram of xenon difluoride (xef₂) lewis diagrams. Carbon (c) will always be the central atom and hydrogen (h) will never be the central atom 2. Web practice sheet electron dot (lewis) structures a lewis or electron dot structure is a convenient representation of the valence electrons in an atom. Web things to remember 1. An electron dot structure for an atom is simply the symbol for the element, surrounded by a number of dots equal to the number of valence electrons. Web draw lewis structures for the following molecular formulas: Practice drawing these lewis structures and don't worry we will go over all the answers step by step. This online quiz is intended to give you extra practice in identifying and drawing lewis dot structures as well as predicting ion formation this quiz aligns with the following ngss standard (s): When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Draw the best lewis dot structures for each of the following species. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Lewis diagram of formaldehyde (ch₂o) worked example:
Lewis Structure Practice Worksheet Lewis Dot Diagrams Chemistry Handout

3 Ways to Draw Lewis Dot Structures wikiHow

Lewis Dot Structure Practice Worksheet

How to Draw a Lewis Structure

Lewis Dot Diagram Practice
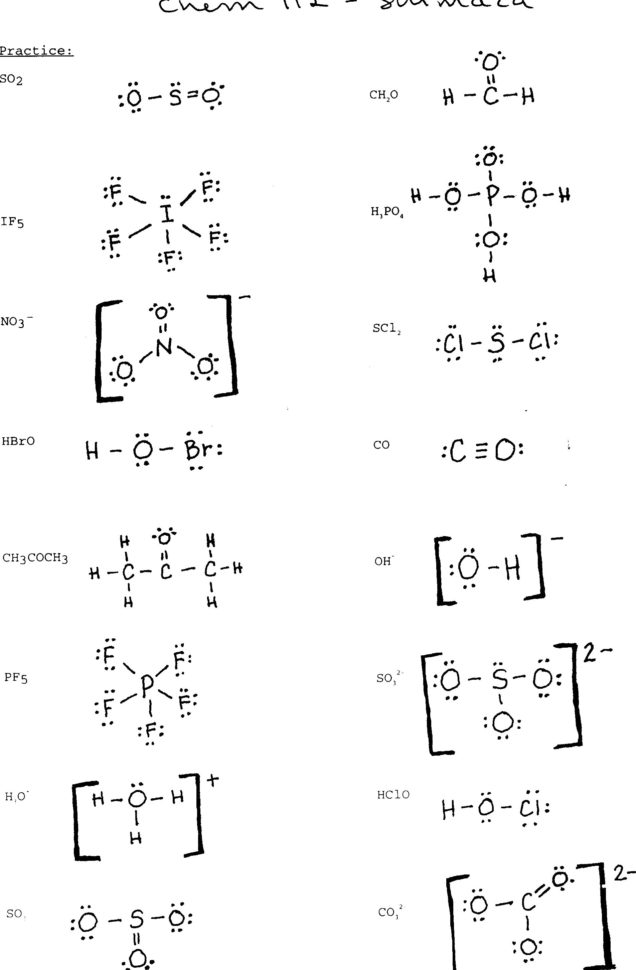
41 more lewis structures worksheet answers Worksheet Works
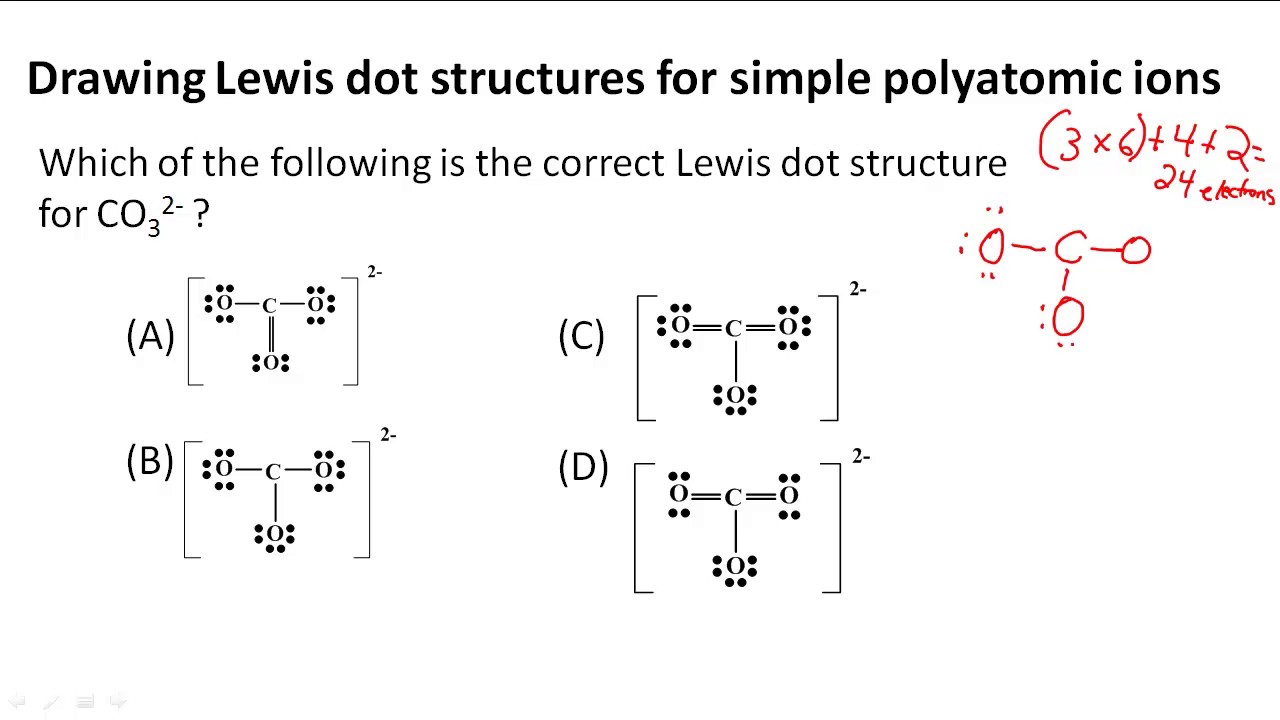
Lewis Structure Practice Worksheet

Lewis Dot Structure Covalent Bonds Worksheets Chemistry worksheets
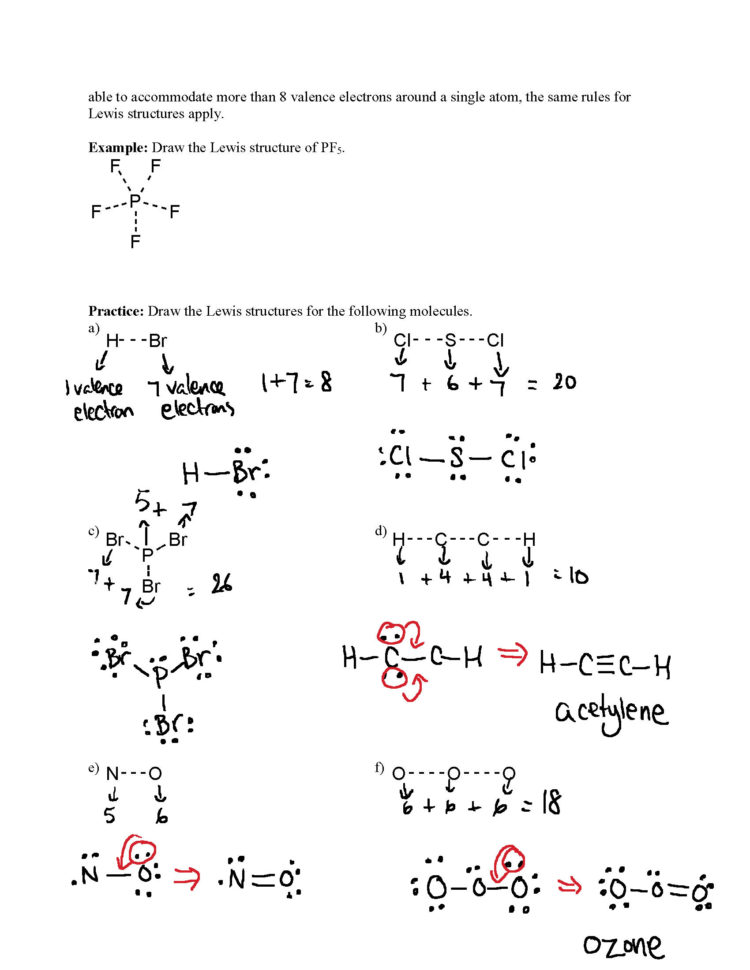
Lewis Structure Worksheet With Answers Handmadefed

Lewis Structure Worksheet With Answers
A Lewis Electron Dot Structure Describes The Bonding Atoms, The Number Of Bonds In The Molecule, And The Lone Pairs Left In The Bonding Atoms.
Web 1 Concept How To Use Organic Chemistry To Make Lewis Structures Easier.
Find The Total Number Of Valence Electrons In This Step, Add Up The Total Number Of Valence Electrons From All The Atoms In The Molecule.
Web Draw The Lewis Dot Structure For Each Of The Following Polyatomic Ions:
Related Post: