Draw Sodium Atom
Draw Sodium Atom - Web in the diagram above, we see a neutral atom of sodium, na, losing an electron. Web this video shows how to draw the orbital diagram of sodium (na). The atomic number of an element is equal to the number of protons and electrons in that element. It is in group 1 of the periodic table. In this video we'll look at the atomic structure, valence electrons,. Web steps here’s how you can draw the bohr model of sodium step by step. Web sodium (na) orbital diagram, electron configuration, and valence electrons. It also shows how to write the electron configuration of sodium (na) and the shorthand nobl. Find the number of protons, electrons, and neutrons in the sodium atom protons are the positively charged particles. For the na+ structure use the periodic table to find the total number of valence electrons for. To draw the bohr model of sodium we must first find out the number of all the atomic particles present in the sodium atom. Sodium is the 11th element of the periodic table so its atomic number is 11. The active atomic mass of the sodium atom is 22.98976928. The atomic number of an element is the number of electrons. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Find the number of protons, electrons, and neutrons in the sodium atom protons are the positively charged particles. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web 28,372 views 294 the sodium atom (na) is commonly used for examples. To illustrate this using an electron dot diagram, we would. First, let us determine the number of protons in the sodium atom. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted zigzag line. Even. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. Web • the atomic mass of sodium is 22.989. The atomic number of an element is the number of electrons and protons in that element. Sodium. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. The element atomic number and name are listed in the upper left. Even a minute amount of water can create this type of reaction. Sodium has the symbol na and “it is the sixth most abundant element in the earth’s crust”. Always write. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web draw a lewis electron dot diagram for an atom or a monatomic ion. A sodium atom has 1 electron in its outer shell. I show you where sodium is on the periodic table and how to determine how. For the na+ structure use the. Web as shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. Web sodium is a chemical element; Web steps to draw the bohr model of sodium atom 1. Always write the atomic number of the element first. Sodium has the symbol na and “it is the sixth most abundant element in the. To draw the bohr model of sodium we must first find out the number of all the atomic particles present in the sodium atom. Web this video shows how to draw the orbital diagram of sodium (na). Web so, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium. #1. Draw the nucleus of an atom a nucleus is a dense and small region that contains the number of protons and neutrons of. #1 write protons, neutrons, and electrons of sodium atom Web properties of sodium atom. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. Sodium is the 11th element of. Web sodium is atomic number 11, and it is a metal, as it lies to the left of the dotted zigzag line. The result is that the sodium ion, na + , has 11 protons, but only 10 electrons. The element atomic number and name are listed in the upper left. In this video we'll look at the atomic. To draw the bohr model of sodium we must first find out the number of all the atomic particles present in the sodium atom. Similarly, neon has a complete outer 2n shell containing eight electrons. The number of protons is always equal to the atomic number of an atom. Atomic number of any element refers to the number of electrons in an atom of that element they are having. Web 131k views 5 years ago. Additionally, sodium is found in group one, which means it has one valence electron. Web sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. Find the number of protons, electrons, and neutrons in the sodium atom protons are the positively charged particles. Web sodium is a chemical element; So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. The atomic number of sodium atoms is 11. The active atomic mass of the sodium atom is 22.98976928. The sodium electron configuration is 1s 2 2s 2 2p 6 3s 1. It is in group 1 of the periodic table. Web for atoms with more than two protons and two electrons, a few rules need to be introduced to diagram them in a way that represents their chemical properties. I show you where sodium is on the periodic table and how to determine how.
FileElectron shell 011 sodium.png Wikimedia Commons
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
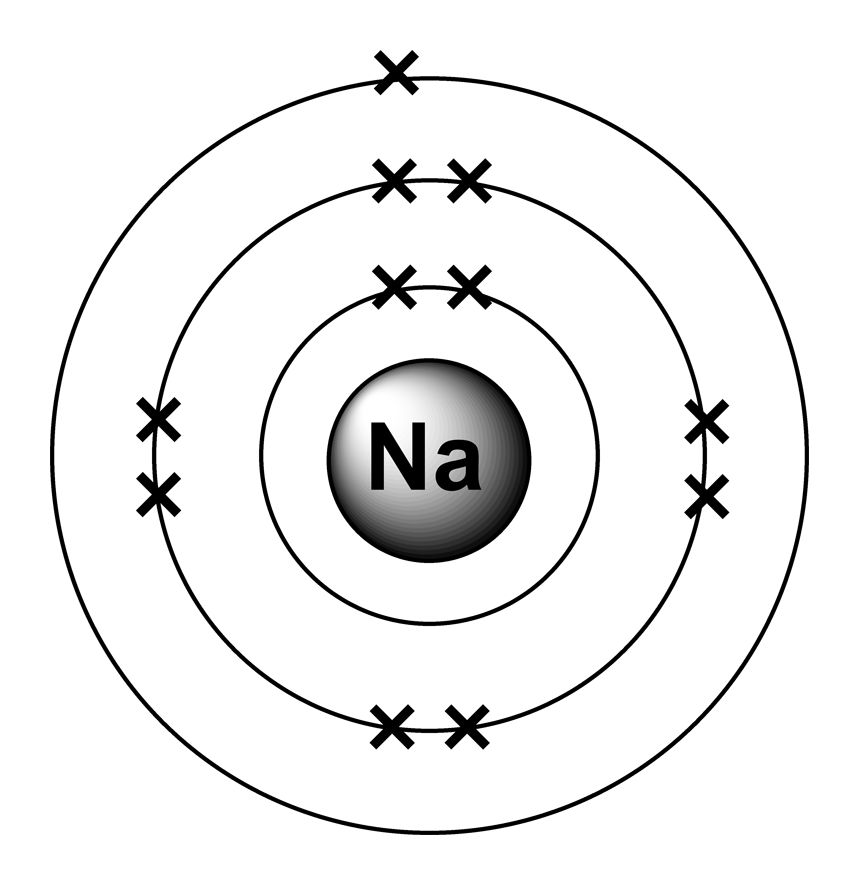
Sodium Table of Elements by Shrenil Sharma
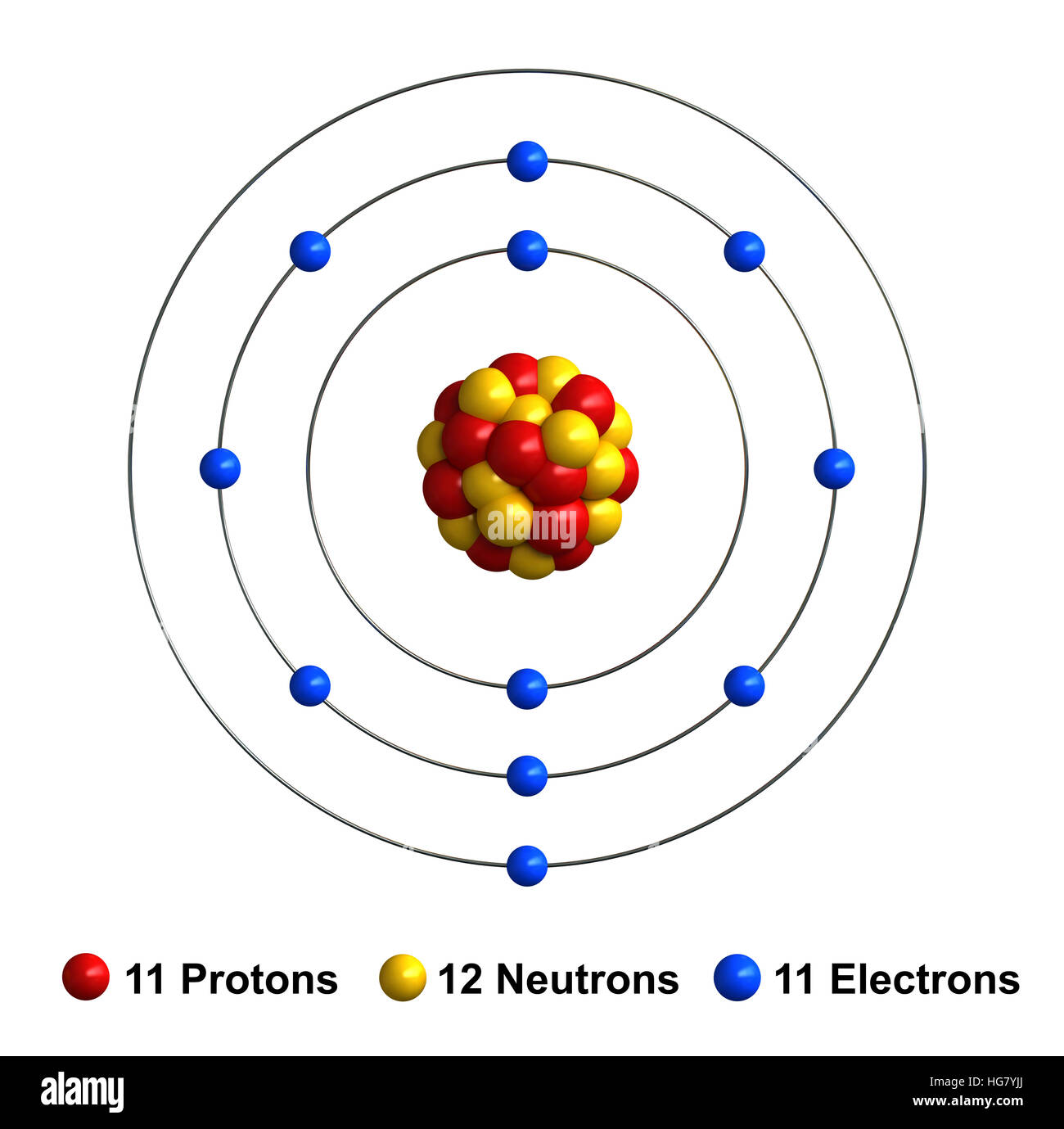
3d render of atom structure of sodium isolated over white background

Sodium Na (Element 11) of Periodic Table NewtonDesk
Chemistry 2.Draw the atomic structure of a sodium atom and a sodium
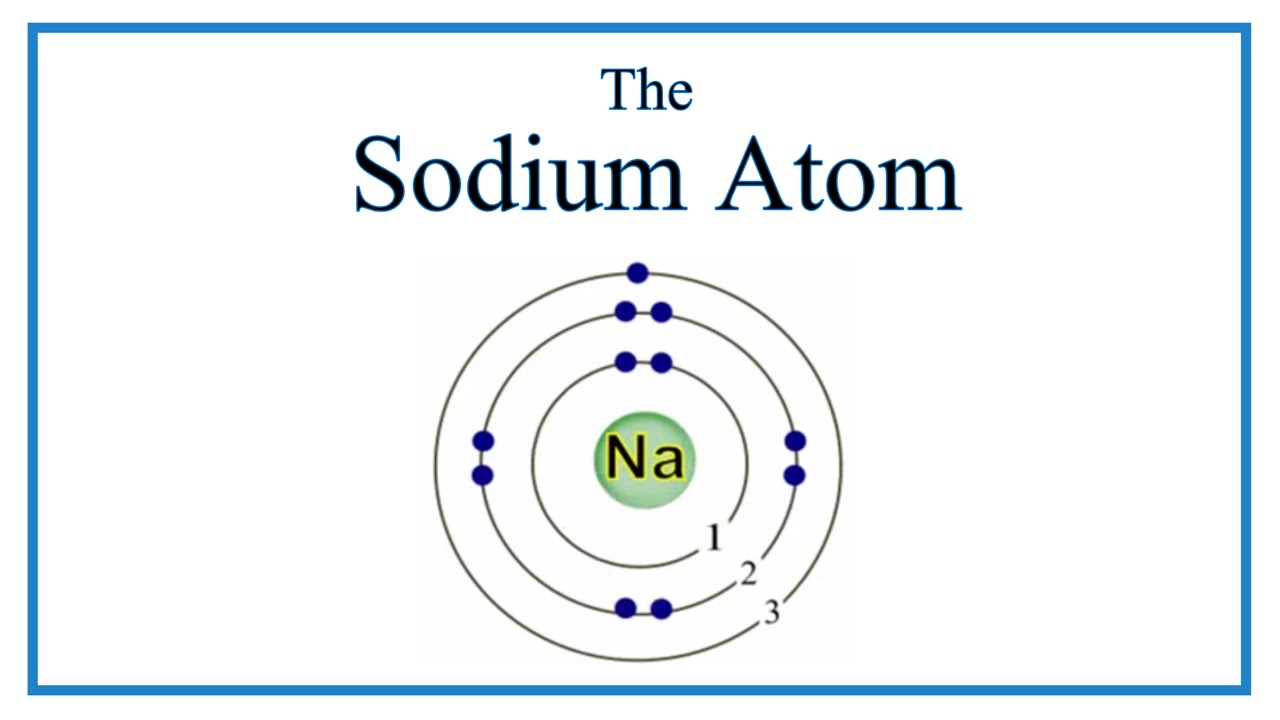
Atomic Structure of the Sodium Atom (Na) YouTube

Diagram representation of the element sodium Vector Image

Sodium atom polizworth

Atom sodium Royalty Free Vector Image VectorStock
Web This Video Shows How To Draw The Orbital Diagram Of Sodium (Na).
Sodium Has An Atomic Number Of 11 Belongs To Group 1 Also Known As The Alkali Metal Group.
Web 28,372 Views 294 The Sodium Atom (Na) Is Commonly Used For Examples And Practice Problems In Chemistry.
Since Sodium Is Number 11 On The Periodic Table We Know That It Contains 11P+.
Related Post: