Draw The Conjugate Acid Of Nh3
Draw The Conjugate Acid Of Nh3 - Draw the conjugate acid for each of the following. Web 2 answers by expert tutors best newest oldest eva a. What is the concentration of h+ in a 0.025 m hcl solution? What is the conjugate acid of nh3? A conjugate acid is formed when a proton is added to a base, and a conjugate base is formed when a proton is removed from an acid. This reaction highly favors the formation of products, so the reaction arrow is drawn only to the right. Conjugate acid of nh 3 the given species is ammonia nh 3. To draw the conjugate acid of each base, we need to add a proton (h+) to each base. The conjugate acid of nh3 is nh4+. Web in order to find the conjugate acid of nh3 we must first understand the bronsted lowery definitions for acids and bases. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Thus nh 3 is called the conjugate base of nh 4 +, and nh 4 + is the conjugate acid of nh 3. Web byju's answer standard xii chemistry bronsted lowry theory what is the c. Let's now look at a reaction involving ammonia,. Nh3 + h+ → nh4+ cl− + h+ → hcl (ch3)2c=o + h+ → (ch3)2choh+ note: Web this species does not exist in water, but it does exist in liquid ammonia. Conjugate acids have one extra proton compared to the species that is forming it. For an acid to be ionized, it must have released a proton. Web 2 answers. Web (a) draw the conjugate acid of | quizlet l l. Nh4+ is strong conjugate acid. Conjugate acid when a proton is added to a base, a conjugate acid is formed. Which segment has a greater length, a x ‾ \overline {ax} ax or b x ‾ \overline {bx} bx? Medium view solution > what is the order of acidic. Web for each of the reactions given below, identify the conjugate acid and the conjugate base. Web solution verified by toppr nh 3 acts as a base as it accepts a hydrogen ion to form nh 4+ ∴ conjugate acid of nh 3 is nh 4+. Web chemistry chemistry questions and answers draw the conjugate base for each of the. Conjugate acids have one extra proton compared to the species that is forming it. Web (a) draw the conjugate acid of | quizlet l l. Both mass and charge are conserved. Which segment has a greater length, a x ‾ \overline {ax} ax or b x ‾ \overline {bx} bx? Medium solution verified by toppr nh 3 acts as a. Web 14.7d | how to find the conjugate acid and conjugate base of nh3 the glaser tutoring company 38.6k subscribers subscribe 590 views 10 months ago chapter 14 | solution manual for chemistry. Web conjugate acids and conjugate bases are the acids and bases that lose or gain protons. This reaction highly favors the formation of products, so the reaction. Nh4 + 2.what is the conjugate acid of the hydroxide ion? Choose a point c c c on l l l but not on a b ‾ \overline {ab} ab. Oh (b) он (c) nh3 (d) h3o+ (e) oh () (g) nh4+ this problem has been solved! 0 0 similar questions a strong acid has a strong conjugate base. Nh4+. Web for each of the reactions given below, identify the conjugate acid and the conjugate base. Draw the conjugate acid for each of the following. Web chemistry chemistry questions and answers 3.35 draw the conjugate acid for each of the following bases 2. Nh4+ is strong conjugate acid. Nh3 + h+ → nh4+ cl− + h+ → hcl (ch3)2c=o +. Web 2 answers by expert tutors best newest oldest eva a. Web what is the conjugate acid of each base. Oh (b) он (c) nh3 (d) h3o+ (e) oh () (g) nh4+ this problem has been solved! The conjugate acid of ammonia is the ammonium ion, nh_4^+. This reaction highly favors the formation of products, so the reaction arrow is. Nh4+ is strong conjugate acid. Both mass and charge are conserved. For an acid to be ionized, it must have released a proton. Web conjugate acids and conjugate bases are the acids and bases that lose or gain protons. Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of. Ammonia has a lone pair of electrons on the nitrogen atom. The conjugate acid of any species, is the original species plus a proton, h^+. Oh (b) он (c) nh3 (d) h3o+ (e) oh () (g) nh4+ this problem has been solved! Web this problem has been solved! Conjugate acid when a proton is added to a base, a conjugate acid is formed. The conjugate acid of nh3 is nh4+. What is the concentration of h+ in a 0.025 m hcl solution? What is the conjugate acid of nh3? Web solution verified by toppr nh 3 acts as a base as it accepts a hydrogen ion to form nh 4+ ∴ conjugate acid of nh 3 is nh 4+. The conjugate acid structure of ammonia will not have the lone pair, instead it wi. The conjugate acid is formed by adding a proton (h+) to the. Which segment has a greater length, a x ‾ \overline {ax} ax or b x ‾ \overline {bx} bx? Web (a) draw the conjugate acid of | quizlet l l. Web 2 answers by expert tutors best newest oldest eva a. It will act as a lewis base and not as a bronsted base. Web what is the conjugate acid of each base.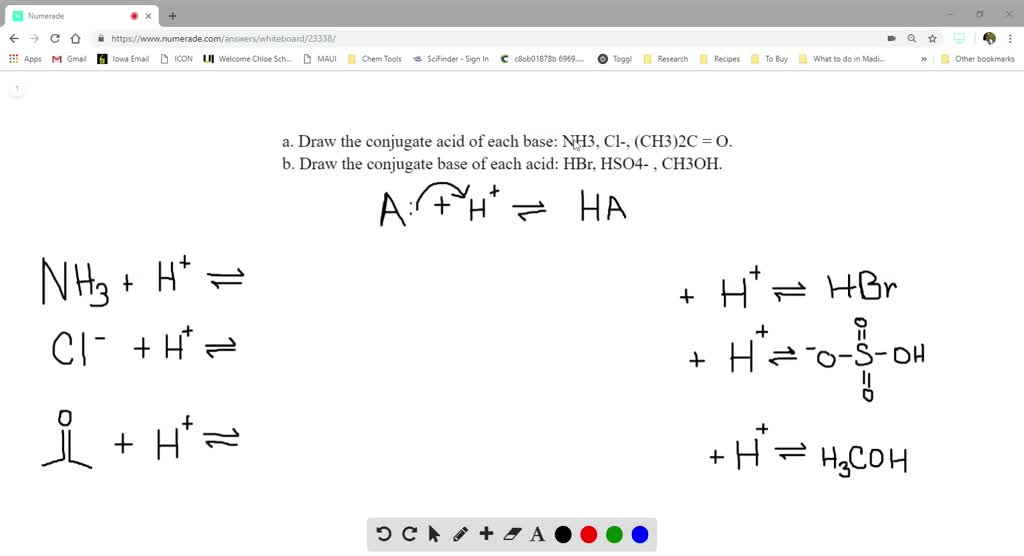
SOLVEDa. Draw the conjugate acid of each base NH3, Cl^, (CH3)2C = O

Solved What is the conjugate the conjuga acid of NH3? as NH₃

Draw the conjugate acid of NH3
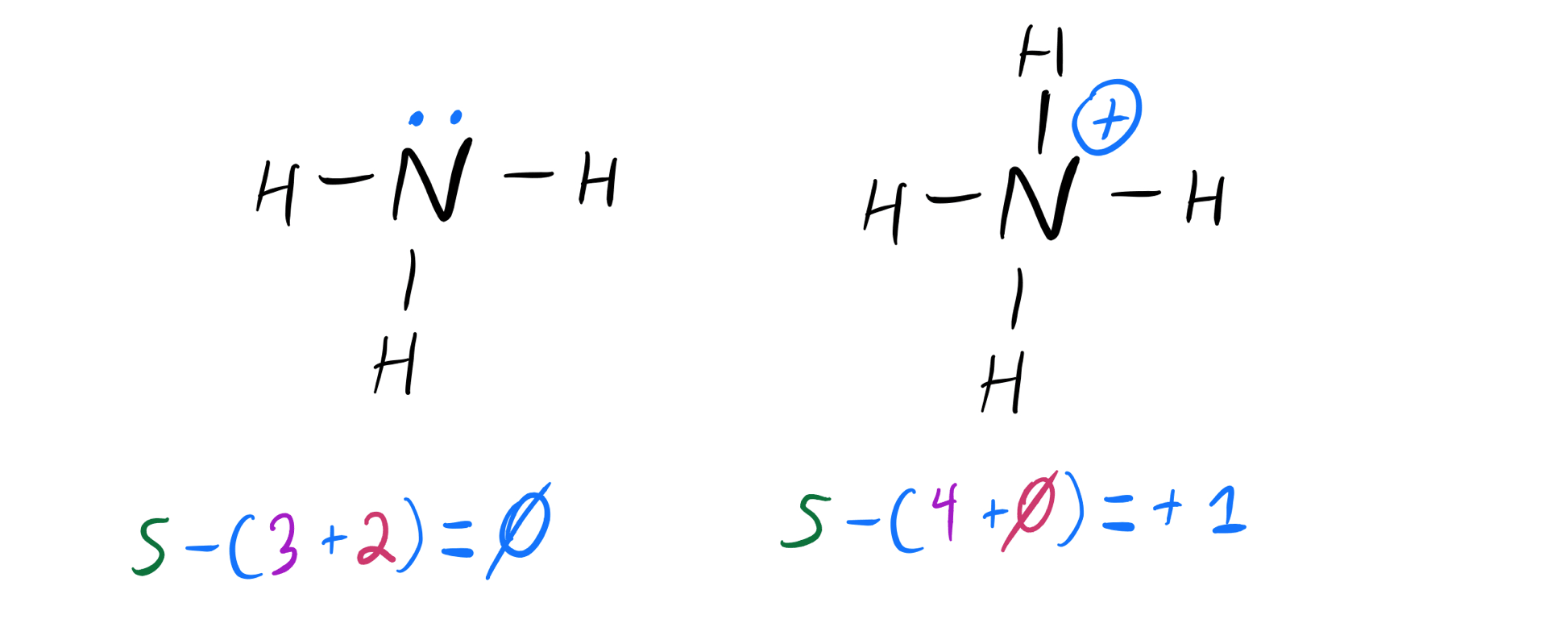
Draw the Lewis Structure for the Conjugate Acid of Ammonia En

Enter the Conjugate Base for Each Acid.

Conjugate Acid of NH3 YouTube
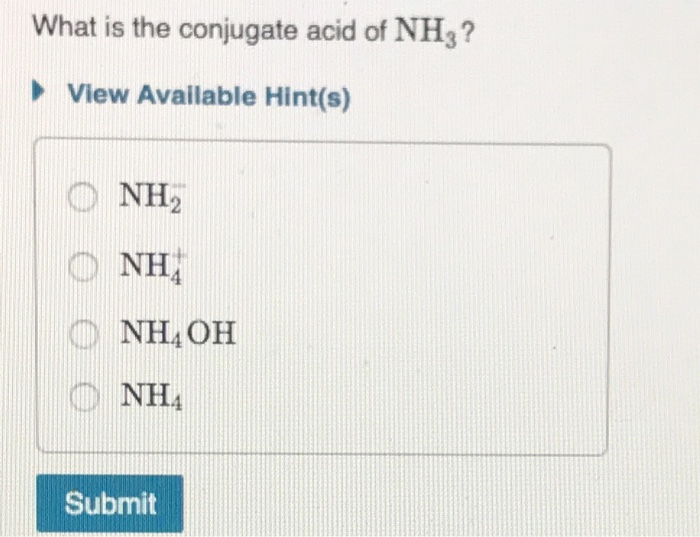
Solved What is the conjugate acid of NH3? View Available

How to draw NH3 Lewis Structure? Science Education and Tutorials

Write the conjugate acids for the following Bronsted bases NH2, NH3
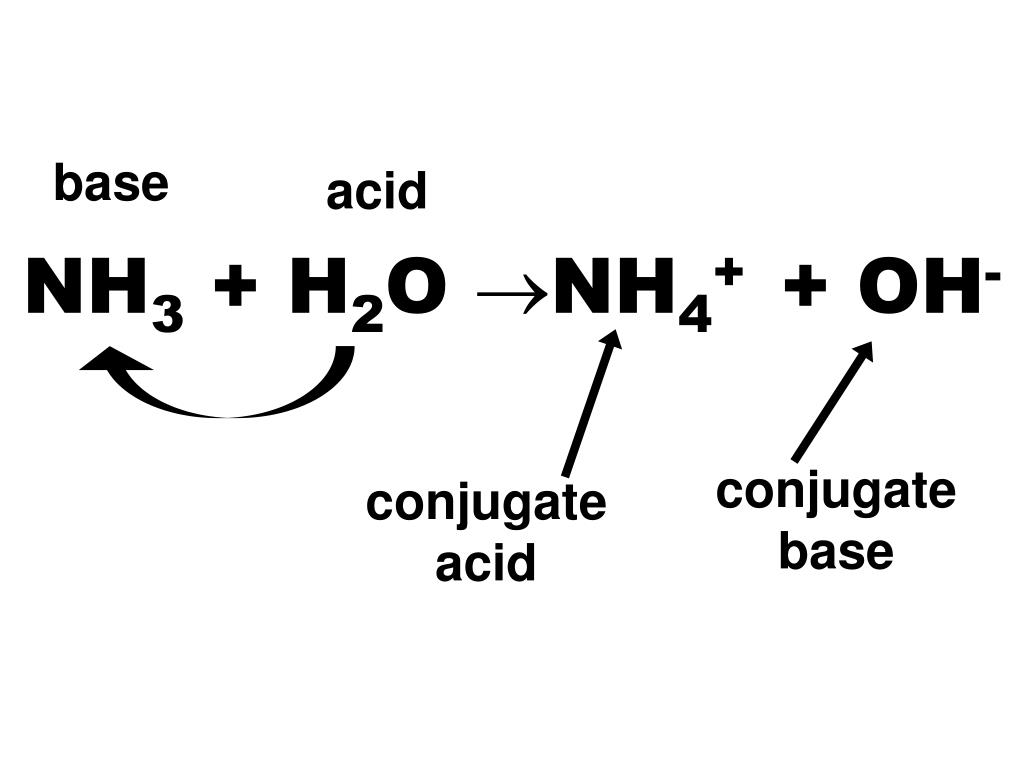
PPT Acid and Base PowerPoint Presentation, free download ID6399344
Call The Point Where L L L And A B ‾ \Overline {Ab} Ab Intersect Point X X X.
Web For Each Of The Reactions Given Below, Identify The Conjugate Acid And The Conjugate Base.
Thus Nh 3 Is Called The Conjugate Base Of Nh 4 +, And Nh 4 + Is The Conjugate Acid Of Nh 3.
Let's Now Look At A Reaction Involving Ammonia, Nh3 , In Water:
Related Post: