Draw The Electron Configuration For A Neutral Atom Of Calcium.
Draw The Electron Configuration For A Neutral Atom Of Calcium. - Put the noble gas in brackets and write the remainder of the electron configuration. There is one unpaired electron. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The helium atom contains two protons and two electrons. Hence, calcium has 2 valence electrons. Web and thus for the neutral atom, we have 20 electrons to distribute: Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the calcium atom. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. This means the first shell (1s) has 2. How. Web the electron configuration and the orbital diagram are: What about potassium, and what about for chlorine? Web what is the electron configuration of a neutral atom of sodium (na)? 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s,. Electron configuration of boron (b) [he] 2s 2 2p 1: Web the electron configuration and the orbital diagram are: Web what is the electron configuration of a neutral atom of sodium (na)? Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2. The atomic number of cl is 17. Sodium (na) has atomic number 11, hence, 11 electrons. In the case of calcium this is 4s2. Web locate the atom on the periodic table. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. 1s 2 2s 2 2p 1: Web calcium has an atomic number of 20. Locate the noble gas element in the period above the element of interest. Web the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). Web the electron configuration and the orbital diagram are: Hence, calcium has. Web the electron configuration of calcium is [ ar] 4s 2 , if the electron arrangement is through orbitals. Lliruns in aiums and molecules drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of calcium. Since 1s can only hold two electrons the next 2 electrons for calcium go in. 1s 2 2s 2 2p 6 3s 1. In the case of calcium this is 4s2. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. The electron configuration follows the order: When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Web calcium has an atomic number of 20. The number of shells shows which period, or row, it’s in and the number of electrons in the outer shell shows which group. Remember, a neutral atom contains the same number of protons and electrons. Calcium has an atomic number of 20, which means it has 20 electrons in a neutral atom. How do you draw the shell diagram of sodium atom? Web this means that the electron configuration for calcium must end with 4s2. Electron configuration through orbit (bohr principle) electron configuration. The next six electrons will go in the 2p orbital. 1s 2 2s 2 2p 2: Energy 0 1 1 x 5 ? Lliruns in aiums and molecules drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of calcium. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2) how do we write. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s,. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2# or simply #ca: For cl atoms, the electron configuration is 3s 2 3p 5. 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (2) how do we write the configuration for ca^ (2+)? There is one unpaired electron. Electron configuration of boron (b) [he] 2s 2 2p 1: Web this means that the electron configuration for calcium must end with 4s2. 1s 2 2s 2 2p 6 3s 1. Sodium (na) has atomic number 11, hence, 11 electrons. Calcium has an atomic number of 20, which means it has 20 electrons in a neutral atom. When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the calcium atom. Web the upper right side shows the number of electrons in a neutral atom. Web draw the electron configuration for a neutral atom of calcium. In the case of calcium this is 4s2. Remember, a neutral atom contains the same number of protons and electrons. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom.
Calcium, atomic structure Stock Image C018/3701 Science Photo Library
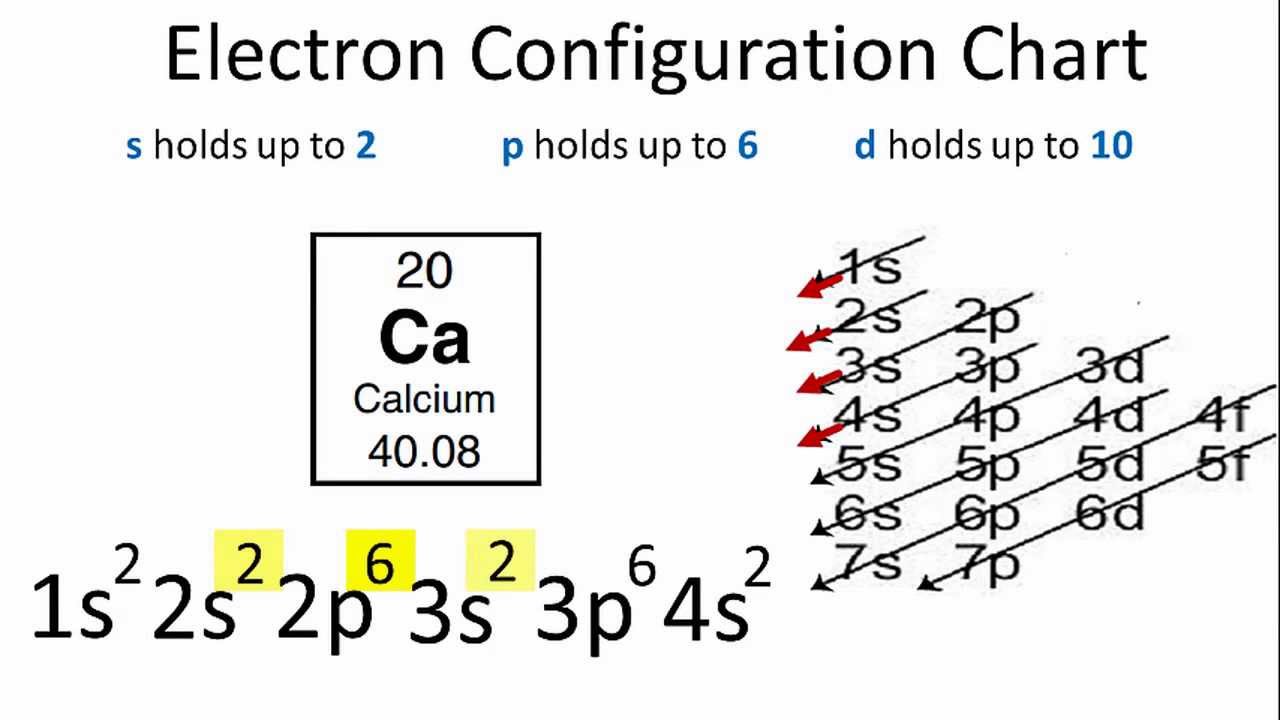
Calcium Electron Configuration (Ca) with Orbital Diagram

Calcium Facts

Bohr Diagram For Calcium

Draw The Electron Configuration For A Neutral Atom, HD Png Download
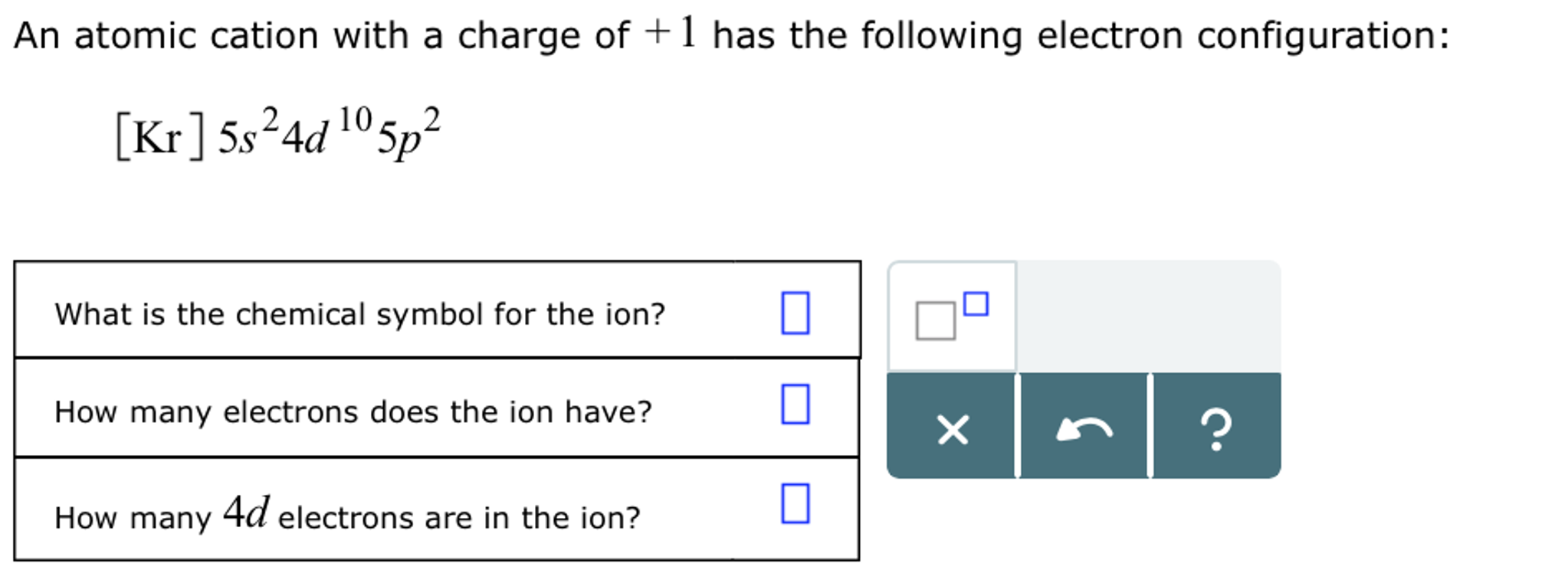
(Get Answer) Draw The Electron Configuration For A Neutral Atom Of
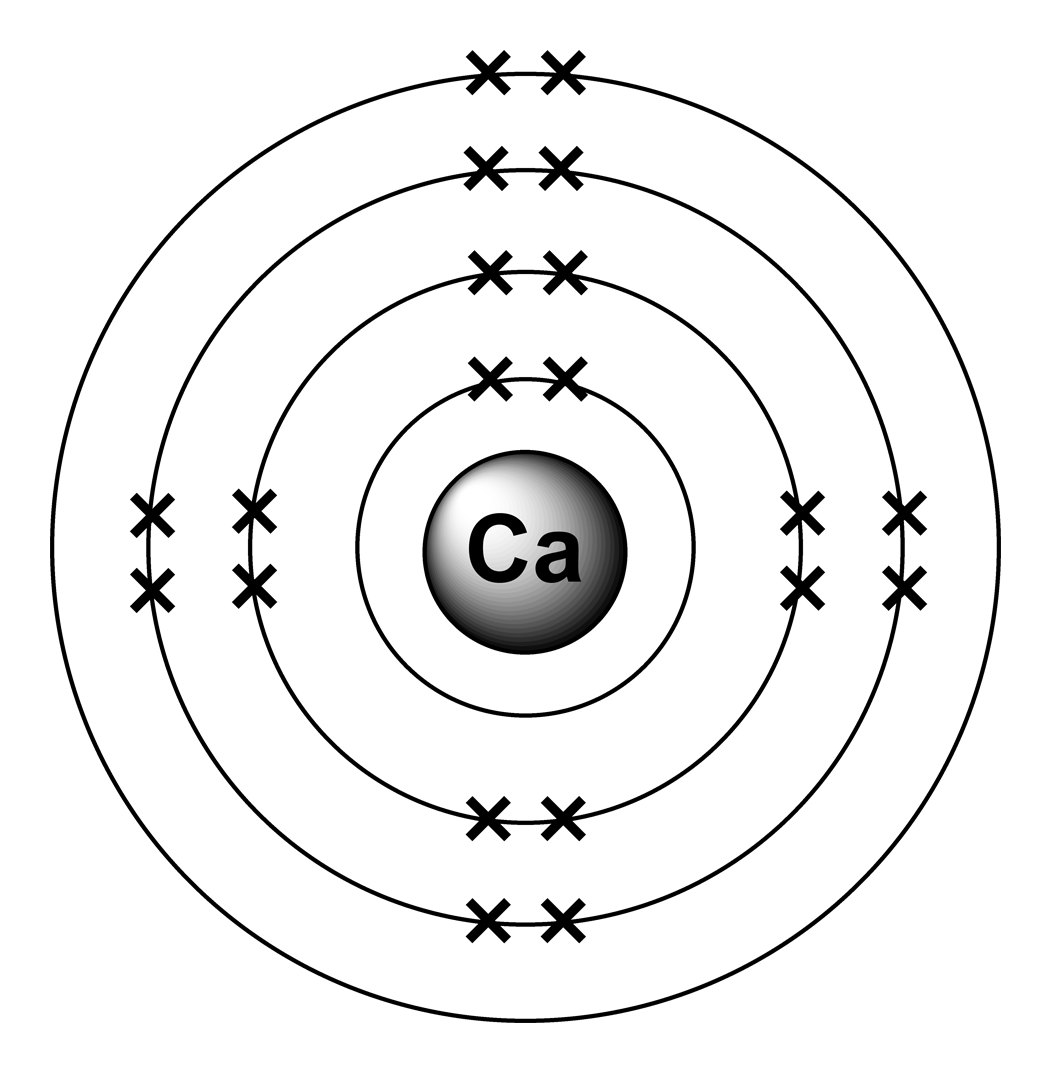
Electron arrangements
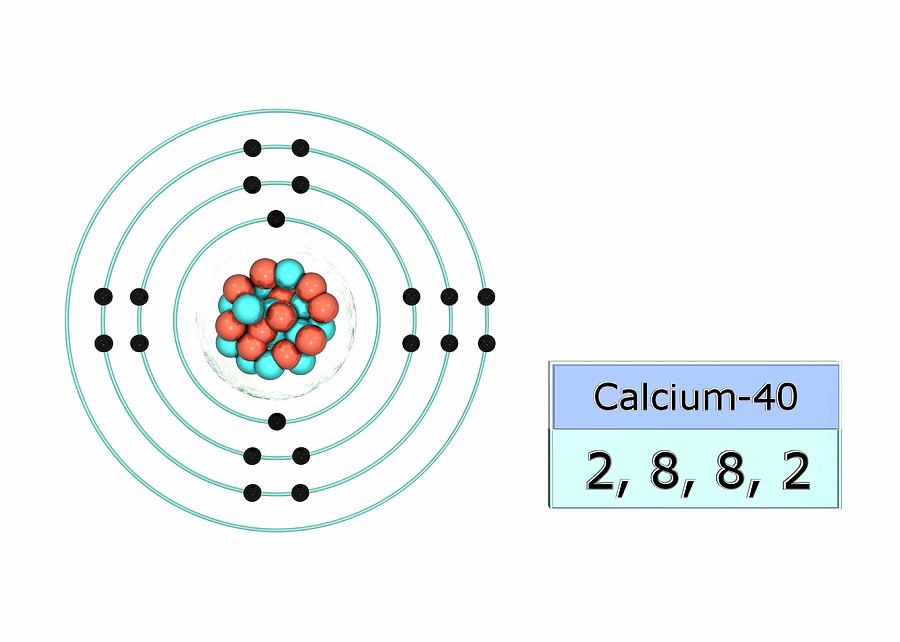
Calcium Electron Dot Diagram Photos Cantik

How Many Valence Electrons are in Calcium Periodic Table

Calcium (Ca) electron configuration and orbital diagram (2023)
Web What Is The Electron Configuration Of A Neutral Atom Of Sodium (Na)?
Energy 0 1 1 X 5 ?
#1S^2, 2 S^2, 2P^6, 3S^2, 3P^4#.
The First Electron Has The Same Four Quantum Numbers As The Hydrogen Atom Electron (N= 1, L= 0, Ml= 0, Ms=+12Ms=+12).
Related Post: