Draw The Electron Configuration For A Neutral Atom Of Neon
Draw The Electron Configuration For A Neutral Atom Of Neon - 1s 2 2s 2 2p 6 3s 1. Now, for the electron configuration of neon, the first 2 electrons will go in 1s orbital since s subshell can hold a maximum of 2 electrons. Web electron configuration of oxygen (o) [he] 2s 2 2p 4: Web a neon atom is a neutral atom that has 10 atomic numbers which imply it has a total of 10 electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web now if we take a neutral atom of neon, ne, which is just to the right of fluorine, neon is practically the opposite of fluorine in terms of reactivity despite both being neutral atoms. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Locate the nearest noble gas preceding phosphorus in the periodic table. Because the second energy level (2s 2 2p 6. This problem has been solved! We continue this path until we reach a total of 10 electrons. If there are more electrons than protons, the ion has a negative charge and is called an anion. Web if there are more protons than electrons, an atomic ion has a positive charge and is called a cation. Otherwise, write the order of. And again this can be explained by neon's valence electron configuration which is:. However, it's easy to determine the configuration of electrons for. If there are more electrons than protons, the ion has a negative charge and is called an anion. Web now if we take a neutral atom of neon, ne, which is just to the right of fluorine,. Answer the electron configuration for a neutral atom of neon is 1s² 2s² 2p⁶. Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. If there are more electrons than protons, the ion has a negative charge and is called an anion. Web neon is the 10th element on the periodic. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Therefore the ne electron configuration will be 1s 2 2s 2 2p 6. Web what is the electron configuration of a neutral atom of sodium (na)? The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web for hydrogen,. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. We find the configuration to be 1s22s22p6 which we know is correct because if you add up the superscripts, you'll get the total number of electrons; You'll. However, it's easy to determine the configuration of electrons for. And again this can be explained by neon's valence electron configuration which is:. Web in writing the electron configuration for neon the first two electrons will go in the 1s orbital. Web unless there is a reason to show the empty higher energy orbitals, these are often omitted in an. Then, since the lithium ion has one less electron, remove an electron from. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. We find the configuration to be 1s22s22p6 which we know is correct because if you add up the superscripts, you'll get the total number of electrons; Draw a small circle and write the symbol in the centre. Web if there are. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. Web now if we take a neutral atom of neon, ne, which is just to the right of fluorine, neon is practically the opposite. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. For example, potassium has 19 electrons. 1s 2 2s 2 2p 4: Web if there are more protons than electrons, an atomic ion has a positive charge and is called a cation. Web unless there is a reason to show the empty. Web neon is the 10th element on the periodic table, meaning it has 10 electrons in its neutral state. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) neon (ne) atom electron configuration through orbitals follows different principles. Web electron configuration of oxygen (o) [he] 2s 2 2p 4: Sodium (na) has atomic number 11, hence, 11. Web how to draw an electron configuration diagram. Web a neon atom is a neutral atom that has 10 atomic numbers which imply it has a total of 10 electrons. #1s^2, 2 s^2, 2p^6, 3s^2, 3p^4#. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. This means there are 2 electrons in the 1s sublevel, 2 electrons in the 2s sublevel, and 6 electrons in the 2p sublevel. The remaining six electrons will go in the 2p orbital. The electron configurations and orbital diagrams of these four elements are: Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 6.8.1 6.8. If there are more electrons than protons, the ion has a negative charge and is called an anion. Web how many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom? Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) neon (ne) atom electron configuration through orbitals follows different principles. Web unless there is a reason to show the empty higher energy orbitals, these are often omitted in an orbital diagram: Web the electron configuration of neon is [ he] 2s 2 2p 6, if the electron arrangement is through orbitals. Draw a small circle and write the symbol in the centre. Orbital diagram and valence electron configuration for phosphorus. Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium).
Electron Configuration for Neon (Ne) Full Explanation
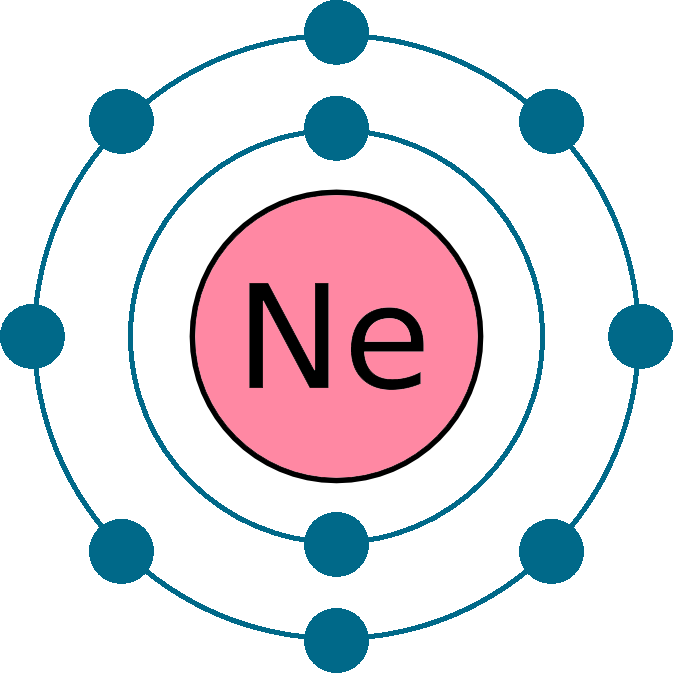
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
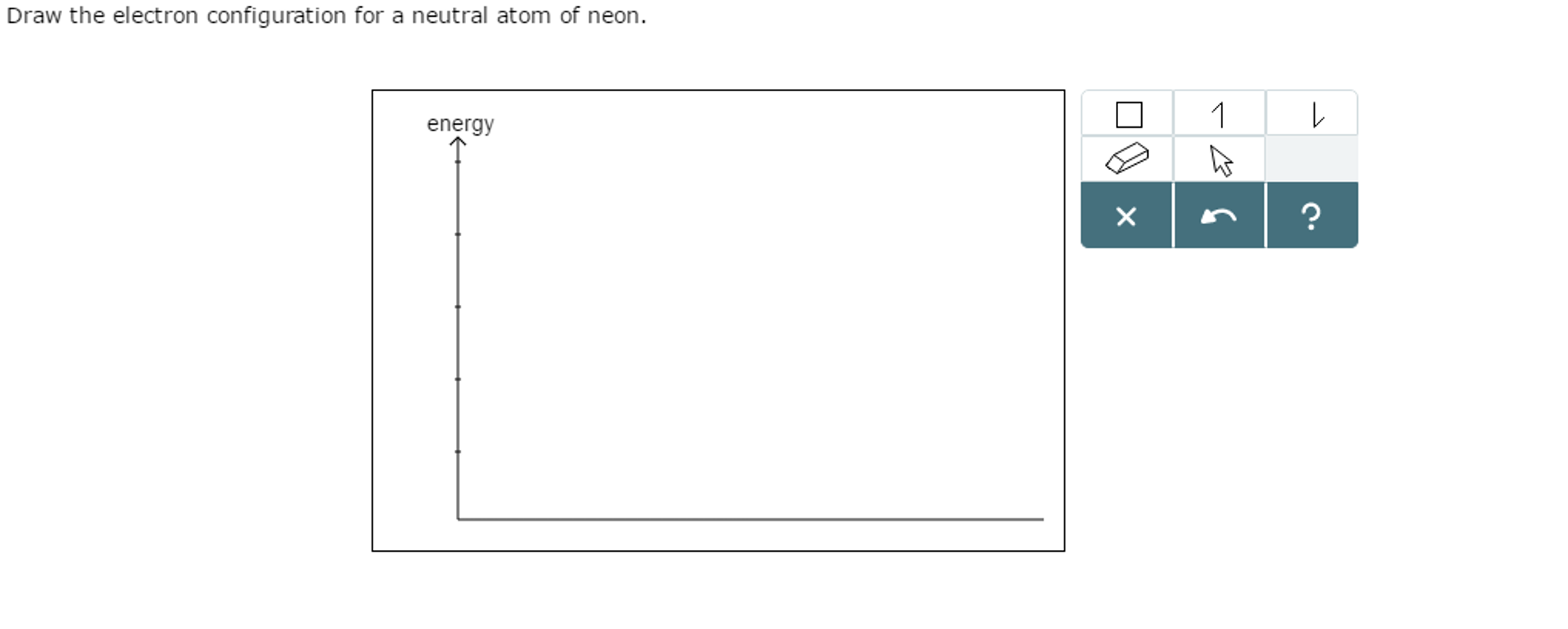
Solved Draw the electron configuration for a neutral atom of

Neon Atom Science Notes and Projects

Diagram representation element neon Royalty Free Vector

Neon Electron Configuration (Ne) with Orbital Diagram

See the Electron Configuration of Atoms of the Elements Neon Atom

Neon (Ne) Electron Configuration YouTube
:max_bytes(150000):strip_icc()/neonatom-58b602755f9b5860464c7e5a.jpg)
Atom Diagrams Electron Configurations of the Elements
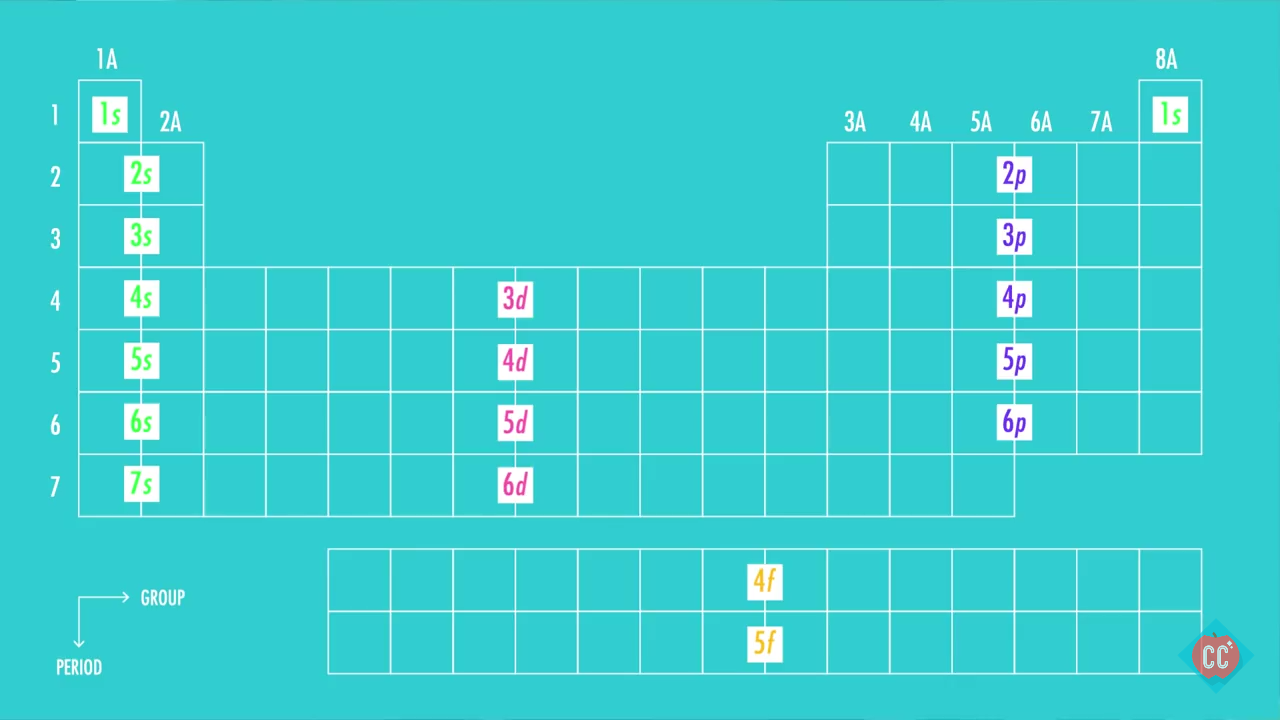
What is the groundstate electron configuration of a neutral atom of
Web Electron Affinity The Energy Released When An Electron Is Added To The Neutral Atom And A Negative Ion Is Formed.
1S 2 2S 2 2P 6 3S 1.
For Example, To Find The Configuration For The Lithium Ion (Li⁺), Start With Neutral Lithium (1S²2S¹).
Sodium (Na) Has Atomic Number 11, Hence, 11 Electrons.
Related Post: