Draw The Electron Configuration For A Neutral Atom Of Nitrogen
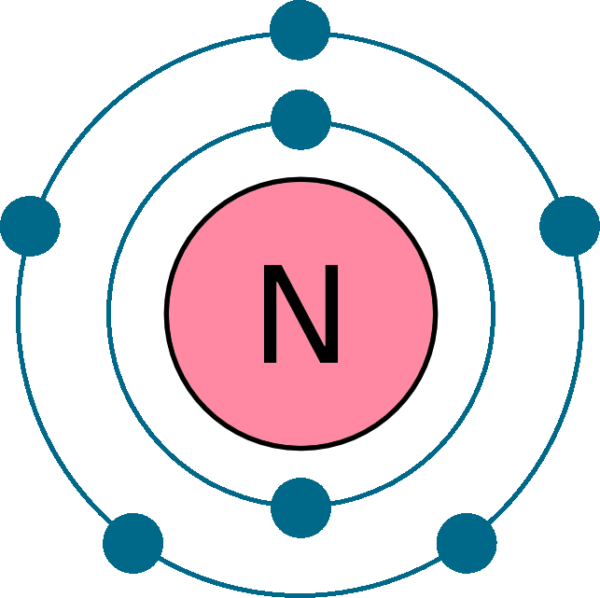
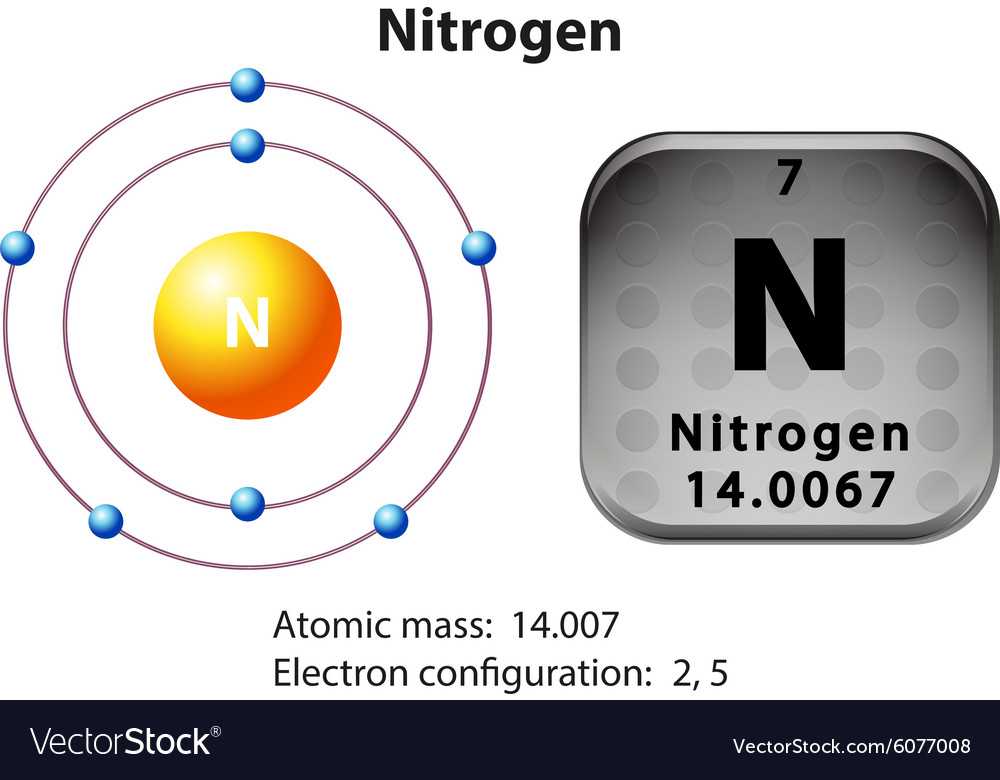
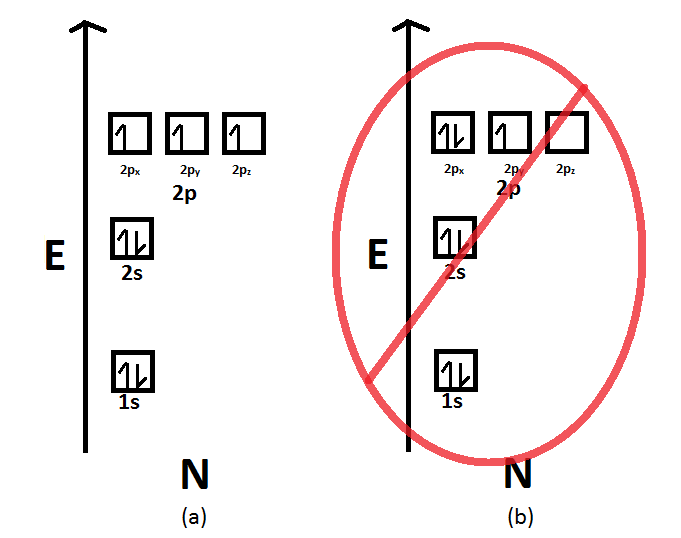
Draw The Electron Configuration For A Neutral Atom Of Nitrogen - The arrangement of an element’s electrons tells you where it is on the periodic table. Web for each electron shell atom diagram, the element symbol is listed in the nucleus. The number of shells shows which period, or row, it’s in and the number of electrons in the outer shell shows which group it’s in. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This makes it easier to understand and predict how atoms will interact to form chemical bonds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with hund’s rule. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. So the electron configuration will include 7 electrons placed into the appropriate s and p orbitals in the ground state (state of lowest energy). Electron configuration of neon (ne) [he] 2s 2 2p 6: 1s 2 2s 2 2p 5: Web electronic configuration of a neutral atom is 1s22s22p3. Web draw the electron configuration for a neutral atom of nitrogen. What is the electron configuration of this atom? Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with hund’s rule. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. 7 electron of the nitrogen are placed. Web the electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. 1s 2 2s. How many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom. What is the name of this atom? 1s 2 2s 2 2p 5: A neutral helium atom, with an atomic number of. The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. Draw an orbital diagram using the shorthand nobel gas configuration and use it to derive the electron configuration of phosphorus, z = 15. The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. The element atomic number and name are listed in the upper left. Web if we look at the correct electron configuration of the nitrogen. Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2. Web the neutral atom chlorine (z=17), for instance has 17 electrons. Web the arrangement of electrons in nitrogen in specific rules in different orbits and orbitals is called the electron configuration of nitrogen. A neutral helium atom, with an atomic number of.. 1s 2 2s 2 2p 6: Web the electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. Energy 1 х this problem has been solved! Electron configuration of fluorine (f) [he] 2s 2 2p 5: Write the electron configuration for a neutral atom of nitrogen. A neutral atom has the same number of electrons as protons. Electron configuration of oxygen (o) [he] 2s 2 2p 4: Web draw the electron configuration for a neutral atom of nitrogen. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Web draw the electron configuration for a neutral atom of nitrogen. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. 1s 2 2s 2 2p 4: The number of shells shows which period, or row, it’s in and the number of electrons in the outer shell shows. 1s 2 2s 2 2p 6: Web for nitrogen this would be 2.5 or 2,5 and for calcium this would be 2.8.8.2 or 2,8,8,2. The atomic number of nitrogen is 7. Web the electron configuration and orbital diagram for carbon are: Electron configuration of neon (ne) [he] 2s 2 2p 6: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. One electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s 2 2 s 2 2 p 4 electron configuration. The number of shells shows which period, or row, it’s in. How many valance electrons are there in the ground state electron configuration of a neutral phosphorus atom. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electron configuration can be done in two ways. Web the electron configuration and orbital diagram for carbon are: Web for example, the ground state electron configuration of nitrogen (1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3) indicates that it has 3 3 3 electrons occupying the 2 p 2 \rm p 2 p orbital. Draw an orbital diagram using the shorthand nobel gas configuration and use it to derive the electron configuration of phosphorus, z = 15. The arrangement of an element’s electrons tells you where it is on the periodic table. Electron configuration of neon (ne) [he] 2s 2 2p 6: Based on hund's rule , one electron fills each p \rm p p orbital, and each electron has the same spin. 1s 2 2s 2 2p 4: Electron configuration of fluorine (f) [he] 2s 2 2p 5: 1s 2 2s 2 2p 6: What is the electron configuration of this atom? Web the arrangement of electrons in nitrogen in specific rules in different orbits and orbitals is called the electron configuration of nitrogen.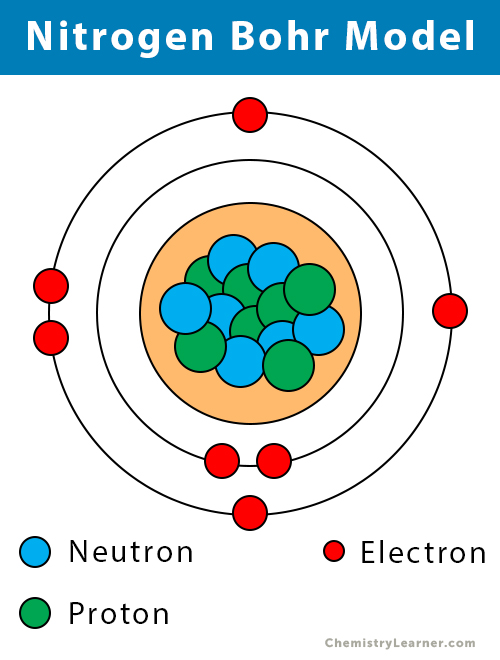
Nitrogen Facts, Symbol, Discovery, Properties, Uses
Nitrogen Element With Reaction, Properties, Uses, & Price Periodic Table

Orbital Diagram For Nitrogen (N) Nitrogen Electron Configuration
Symbol and electron diagram for nitrogen Vector Image
Electron Configuration Chemistry LibreTexts

Diagram representation of the element nitrogen Vector Image
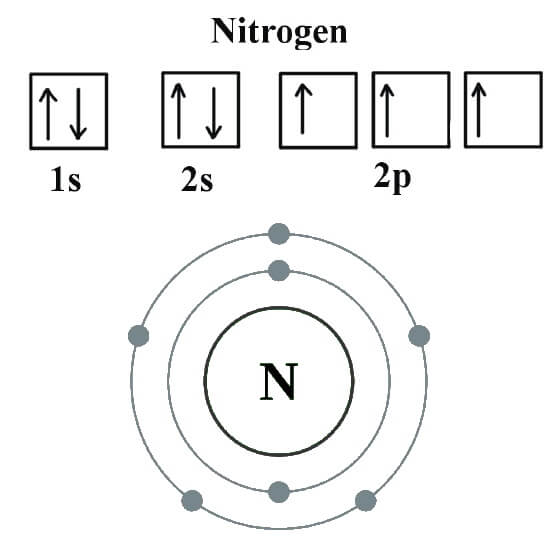
How many valence electrons does nitrogen have? Ask4Essay

How to write the Electronic Configuration of Nitrogen Chemical
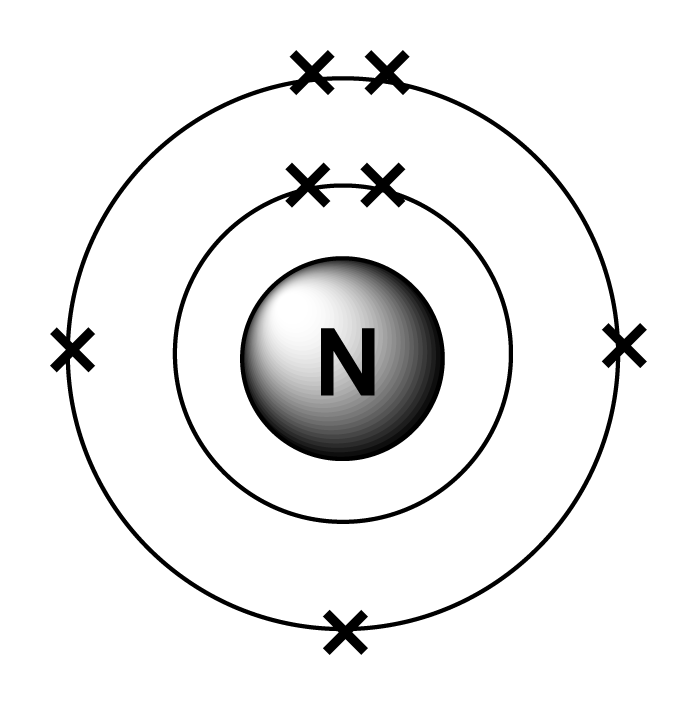
Nitrogen Table of Elements by Shrenil Sharma
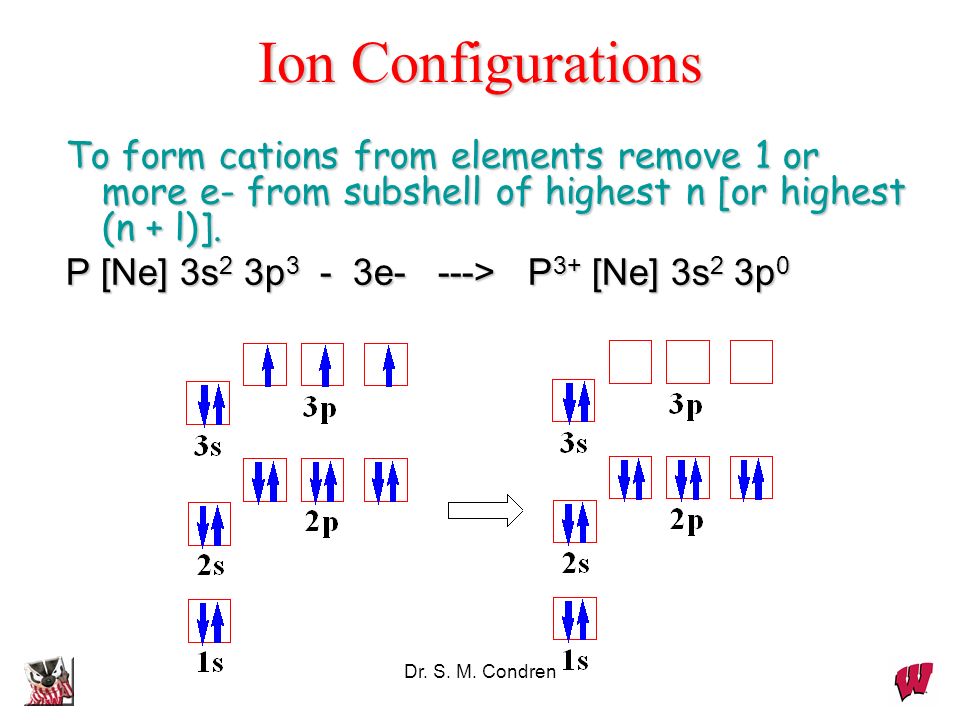
Nitrogen Electron Configuration (N) with Orbital Diagram
Determine Whether The Nitrogen Atom Is Paramagnetic Or Diamagnetic.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web Electron Configuration Of Nitrogen (N) [He] 2S 2 2P 3:
This Problem Has Been Solved!
Related Post: