Draw The Electron Configuration For A Neutral Atom Of Oxygen
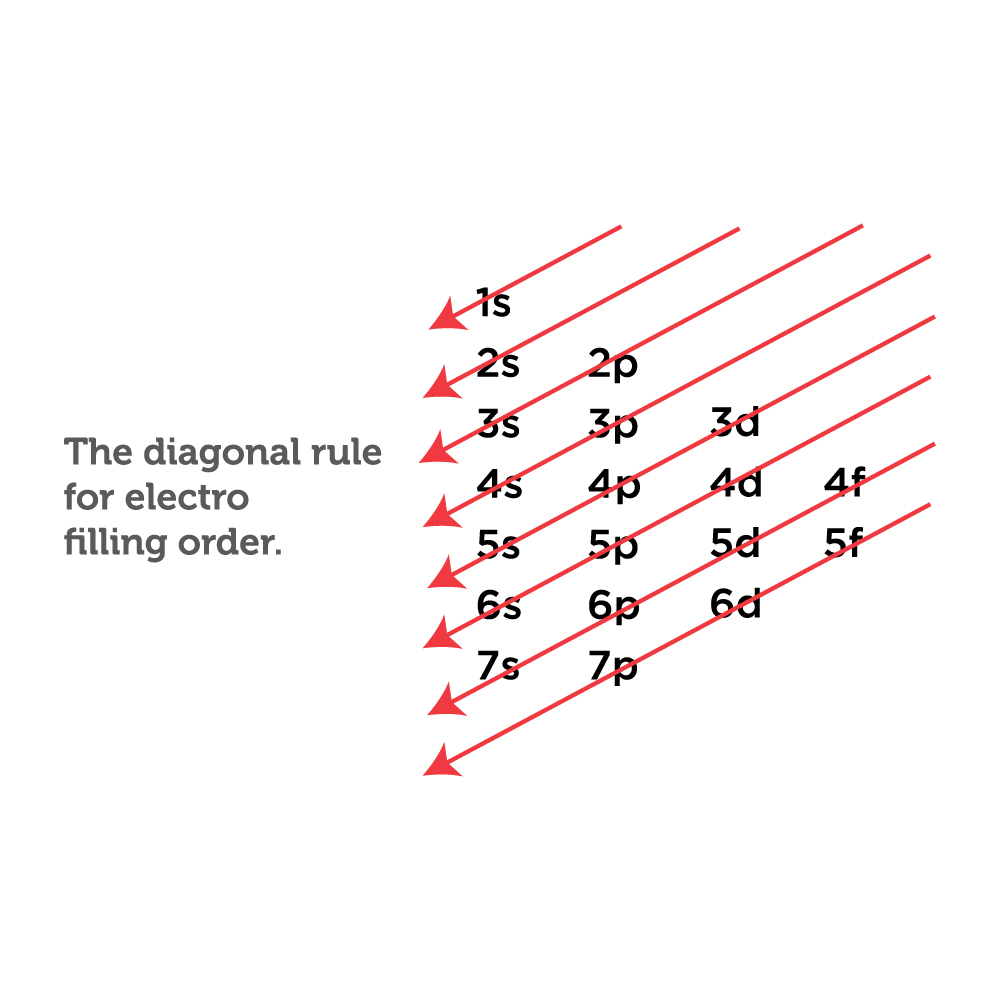
Draw The Electron Configuration For A Neutral Atom Of Oxygen - We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Oxygen has one more electron than nitrogen and as the orbitals are all half filled the electron must pair up. 1s 2 2s 2 2p 4 (for an atom). Web faq this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Since 1s can only hold two electrons the next 2 electrons for o go in the 2s orbital. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Electron configuration of carbon (c) [he] 2s 2 2p 2: Electron configuration of oxygen (o) [he] 2s 2 2p 4: 1s 2 2s 2 2p 6 3s 2 3p 0. Write the configuration of the neutral atom. This allows each halogen atom to have a noble gas electron configuration. Web what is the electron configuration of a neutral atom of sodium (na)? We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet: The given element is oxygen ( o ). 1s 2 2s 2 2p 1: Sodium (na) has atomic number 11, hence, 11 electrons. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Determine how many electrons were lost. Electron configuration of nitrogen (n) [he] 2s 2 2p 3: Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule. One electron must be paired with another in one of the 2 p orbitals, which gives us two unpaired electrons and a 1 s2 2 s2 2 p4 electron configuration. In order to write the o electron configuration we first need to know t. We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet: In order to write the o. Web write lewis symbols for neutral atoms and ions; Draw lewis structures depicting the bonding in simple molecules; The electron configuration of nitrogen is thus 1 s2 2 s2 2 p3. We add electrons to fill the outermost orbital that is occupied, and then add more electrons to the next higher orbital. Web electron configuration of boron (b) [he] 2s. View the full answer step 2. The given element is oxygen ( o ). Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. Sodium (na) has atomic number 11, hence, 11 electrons. Web if we look at the element after nitrogen in the same period, oxygen (z = 8) its. The colors of an aurora are attributed to electron transitions in atomic oxygen and in molecular nitrogen. 1s 2 2s 2 2p 6 3s 1. In order to write the o electron configuration we first need to know t. Write the configuration of the neutral atom. Since 1s can only hold two electrons the next 2 electrons for o go. Electron configuration can be done in two ways. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. Electron configuration of oxygen (o) [he] 2s 2 2p 4: Write the configuration of the neutral atom. And valence electrons are those electrons found in the outer shell of an atom. 1s 2 2s 2 2p 6 3s 2 3p 2. This allows each halogen atom to have a noble gas electron configuration. Draw a lewis structure, showing all bonding and nonbonding electrons, for the oxygen molecule and for the nitrogen molecule. 1s 2 2s 2 2p 6 3s 1 The electron configuration of nitrogen is thus 1 s2 2 s2. Web the arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. Web with three unpaired electrons. The given element is oxygen ( o ). Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals. And valence electrons are those electrons found in the outer shell of an atom. For example, the electron configuration of oxygen looks like: From the pauli exclusion principle, we know that an orbital can contain two electrons with. What is the name of this atom? Electron configuration can be done in two ways. The neutral atom chlorine (z=17), for instance has 17 electrons. 1s 2 2s 2 2p 6 3s 1. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. Web with three unpaired electrons. The given atom is neutral. Sodium (na) has atomic number 11, hence, 11 electrons. 1s 2 2s 2 2p 6 3s 2 3p 2. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Draw lewis structures depicting the bonding in simple molecules; 1s 2 2s 2 2p 4 (for an atom). In order to write the o electron configuration we first need to know t.
What is the Electron Configuration of Oxygen Archives Dynamic
Forms of Energy ND Studies Energy Curriculum
How to Write Ground State Electron Configuration in Chemistry

Electron Configuration Chart With Orbitals
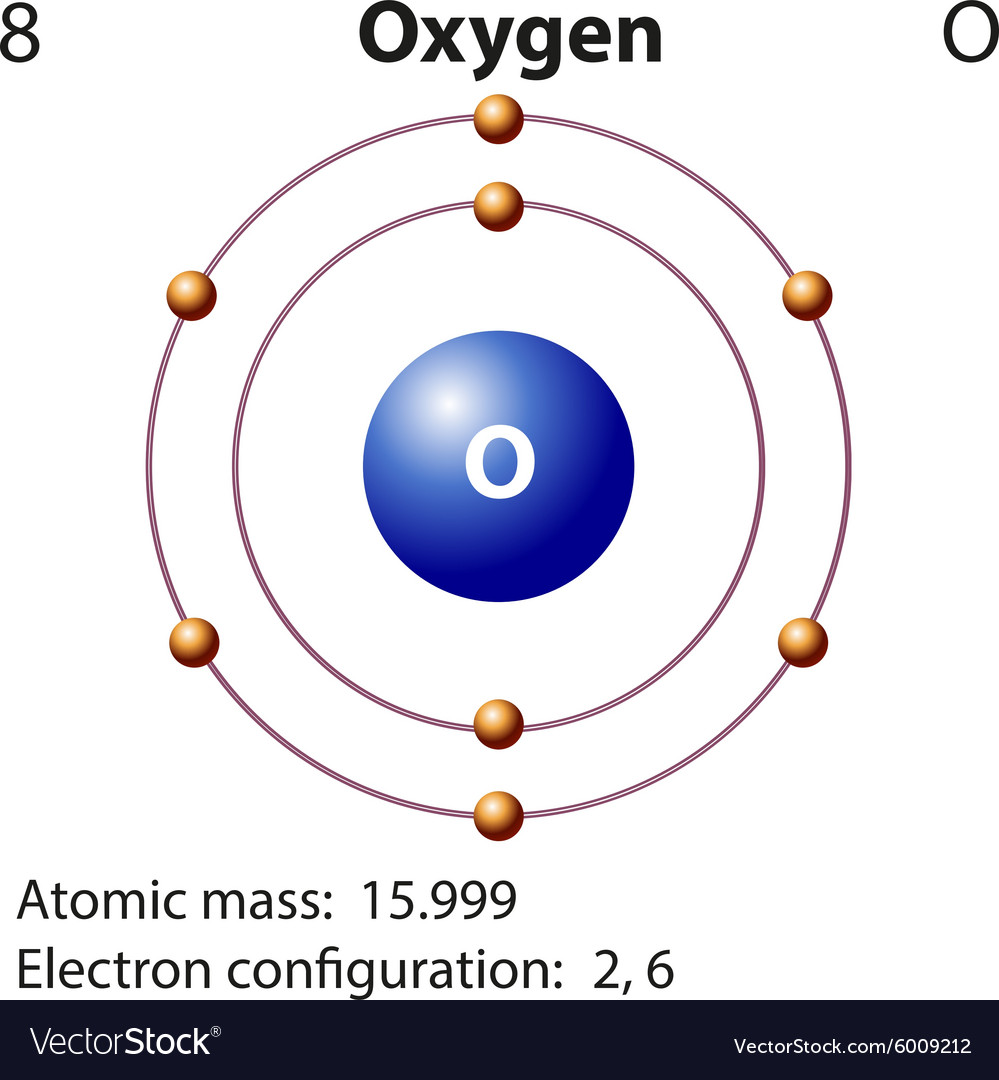
Diagram representation element oxygen Royalty Free Vector
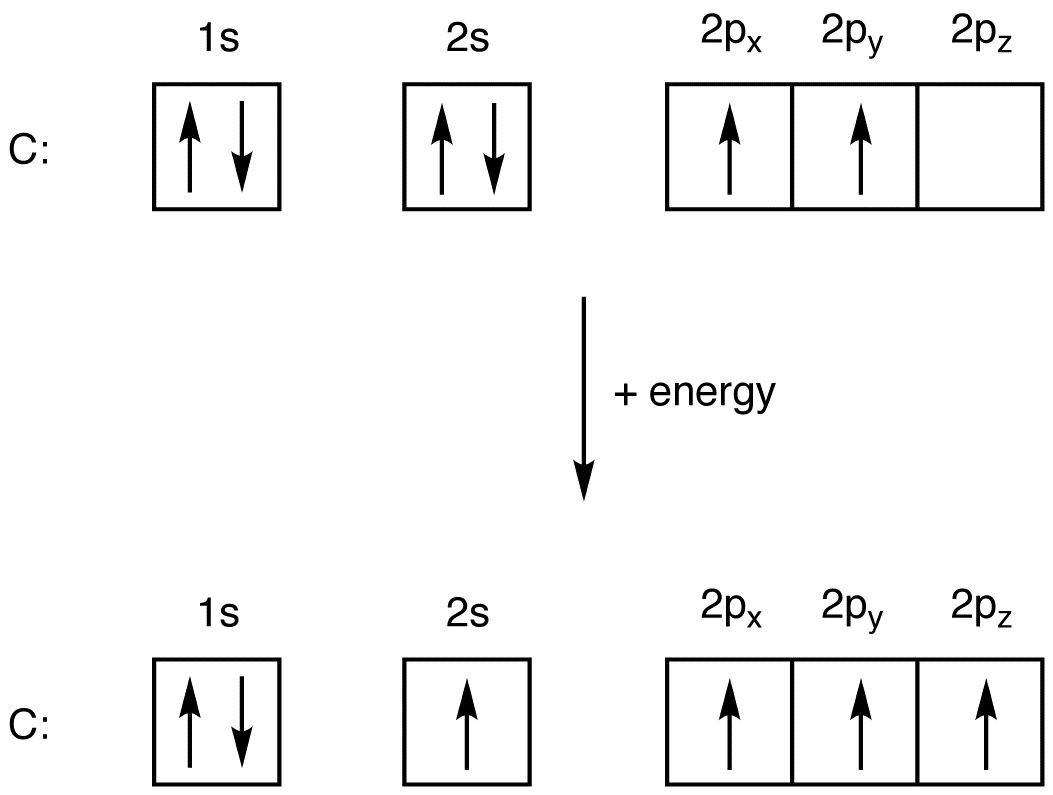
Oxygen Electron Configuration (O) with Orbital Diagram

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
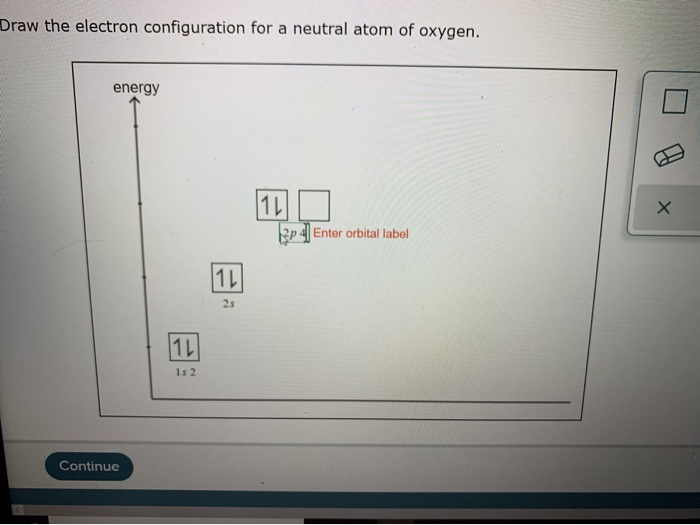
Solved Draw the electron configuration for a neutral atom of
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px

Drawing Atoms Montessori Muddle
Write The Electron Configuration For A Neutral Oxygen Atom And For A Neutral Nitrogen Atom.
Web Faq This Electron Configuration Calculator Will Instantly Show You The Distribution Of Electrons In The Orbitals Of Any Periodic Element You Choose.
Since 1S Can Only Hold Two Electrons The Next 2 Electrons For O Go In The 2S Orbital.
1S 2 2S 2 2P 3:
Related Post: