Draw The Electron Configuration For A Neutral Atom Of Phosphorus
Draw The Electron Configuration For A Neutral Atom Of Phosphorus - Write the electron configuration for a neutral atom of phosphorus. There are 2 steps to solve this one. Draw the electron configuration for a neutral atom of phosphorus. To draw lewis structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Show transcribed image text expert answer transcribed image text: Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. The element atomic number and name are listed in the upper left. This question hasn't been solved yet ask an expert question: Web electron affinity the energy released when an electron is added to the neutral atom and a negative ion is formed. A neutral phosphorus atom has 15 electrons. For the transition metals, electrons are removed from the s orbital first and then from the d orbital. Web the p orbital can hold up to six electrons. Write the electron configuration. Draw the electron configuration for a neutral atom of phosphorus. Phosphorus has 5 valence electrons. Experts have been vetted by chegg as specialists in this subject. First ionisation energy the minimum energy required to remove an electron from a neutral atom in its. Web science chemistry chemistry questions and answers draw the electron configuration for a neutral atom of phosphorus. Phosphorus has 5 valence electrons. Write the electron configuration for a neutral atom of phosphorus. Phosphorus is situated in group 15th or 5a and has an atomic number of 15. Remember, a neutral atom contains the same number of protons and electrons. Web electron affinity the energy released when an electron is added to the neutral atom and a negative. Web write the electron configuration for a neutral atom of phosphorus. Phosphorus has 5 valence electrons. Remember, a neutral atom contains the same number of protons and electrons. This problem has been solved! Web first, write the electron configuration for the neutral atoms: To form bonds with the five chlorine atoms in phosphorus pentachloride, phosphorus utilizes its three 3p orbitals and two 3s orbitals. Web what is the electron configuration and orbital diagram for a phosphorus atom? Write the electron configuration for a neutral atom of phosphorus. Next, remove electrons from the highest energy orbital. Of those 5 electrons, 2 can go into. Web science chemistry chemistry questions and answers write the electron configuration for a neutral atom of phosphorus. Web the shorthand electron configuration for phosphorus is [ne] 3s 2 3p 3. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Experts have been vetted by chegg as specialists in. To draw lewis structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. Phosphorus is situated in group 15th or 5a and has an atomic number of 15. Web the p orbital can hold up to six electrons. Next, remove electrons from the highest energy orbital. Draw the electron configuration. Web the shorthand electron configuration for phosphorus is [ne] 3s 2 3p 3. To draw lewis structures for elements, the symbol for the element is drawn with the number of valence electrons it has surrounding it. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web electron. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Remember, a neutral atom contains the same number of protons and electrons. The number of valence electrons available for the phosphorus atom is 5. The shorthand. Electron configuration can be done in two ways. Web a neutral phosphorus atom has 15 electrons. Each chlorine atom contributes one electron to form a covalent bond with phosphorus. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Web what is the electron configuration and orbital diagram for. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3. Thus, a phosphorus atom contains 15 electrons. Web write the electron configuration for a neutral atom of phosphorus. Web the electron configuration of phosphorus in its ground state is 1s2 2s2 2p6 3s2 3p3. Write the electron configuration for a neutral atom of phosphorus. This means that a neutral phosphorus atom will have 15 electrons surrounding its nucleus. Electron configuration can be done in two ways. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) phosphorus (p) atom electron configuration (bohr model) Web the shorthand electron configuration for phosphorus is [ne] 3s 2 3p 3. Web first, write the electron configuration for the neutral atoms: Web march 23, 2023 by jay electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the. Web the final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The number of valence electrons available for the phosphorus atom is 5. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. The element atomic number and name are listed in the upper left.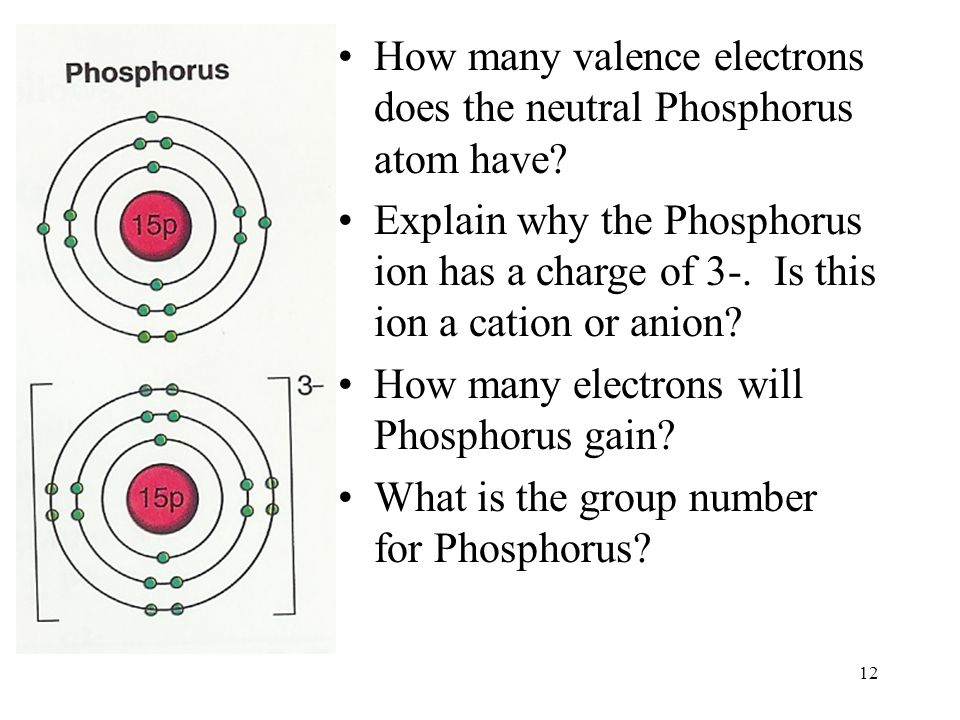
Periodic Table Phosphorus Valence Electrons Periodic Table Timeline
:max_bytes(150000):strip_icc()/phosphorusatom-58b6025c5f9b5860464c65dc.jpg)
Atom Diagrams Electron Configurations of the Elements

Number Of Valence Electrons In Phosphorus
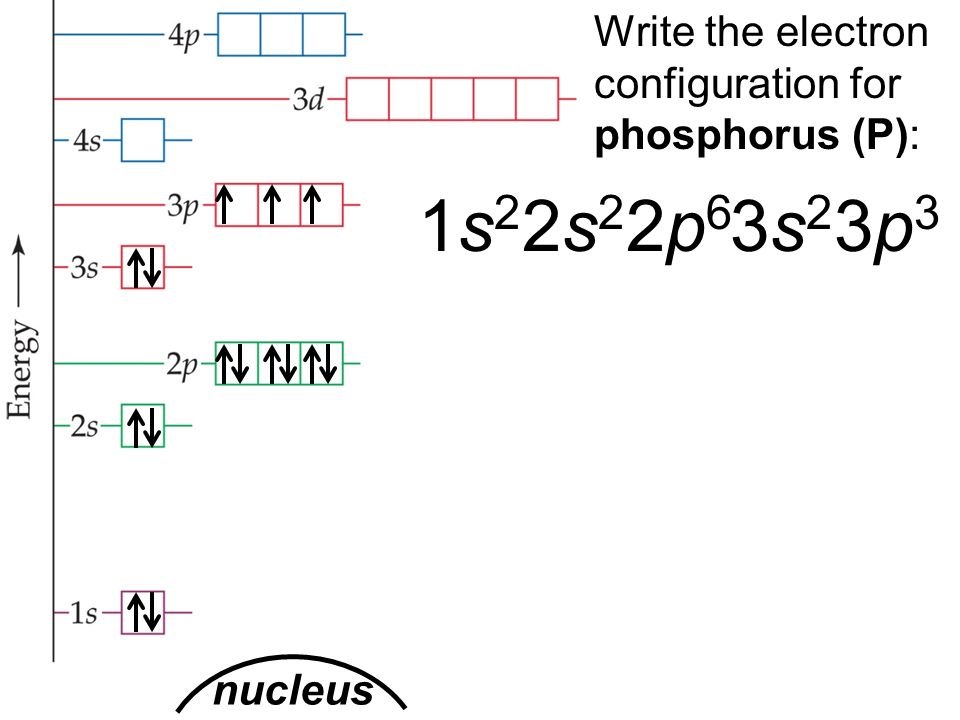
Phosphorus Electron Configuration (P) with Orbital Diagram
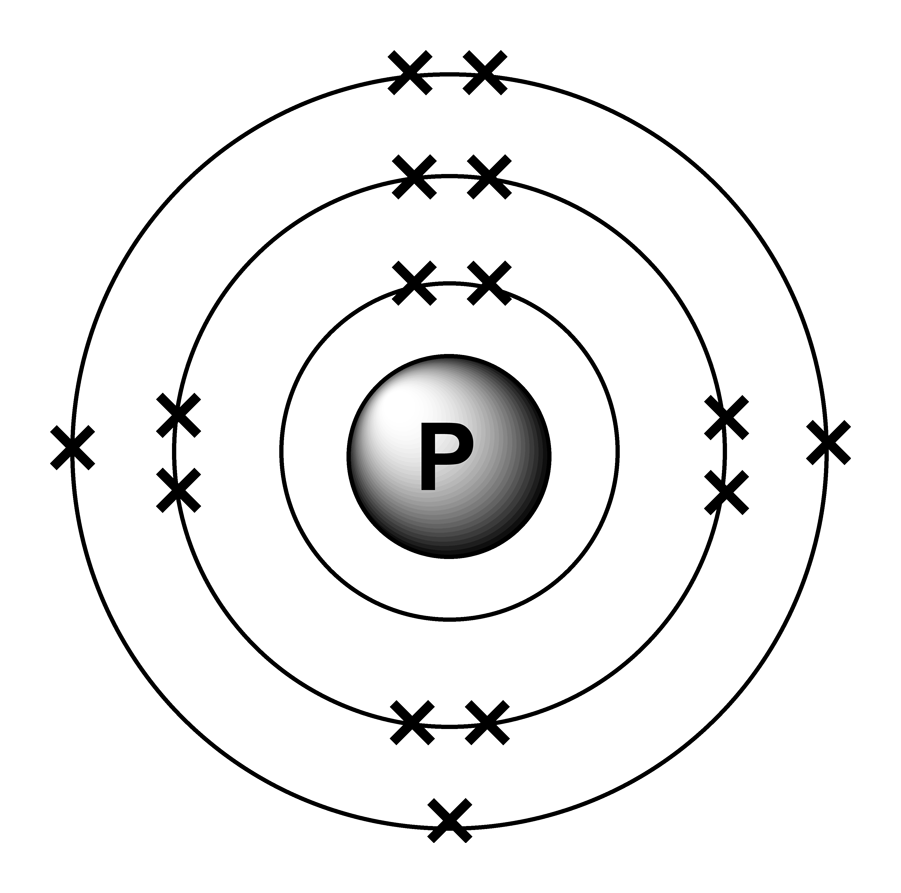
Electron arrangements

Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
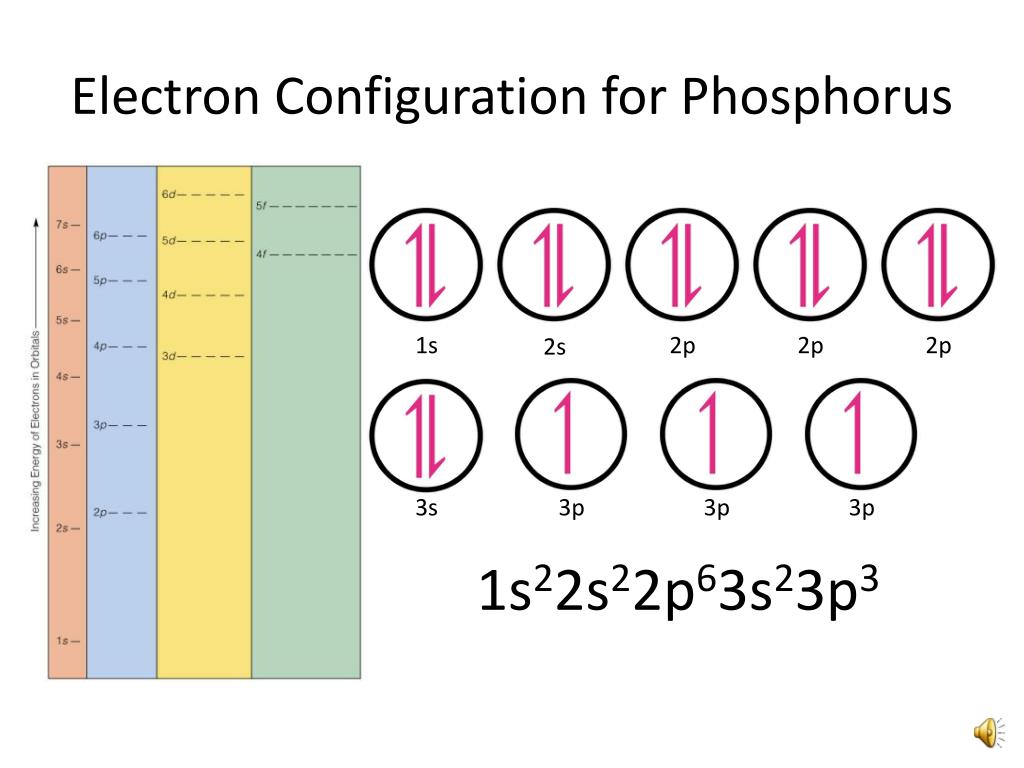
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
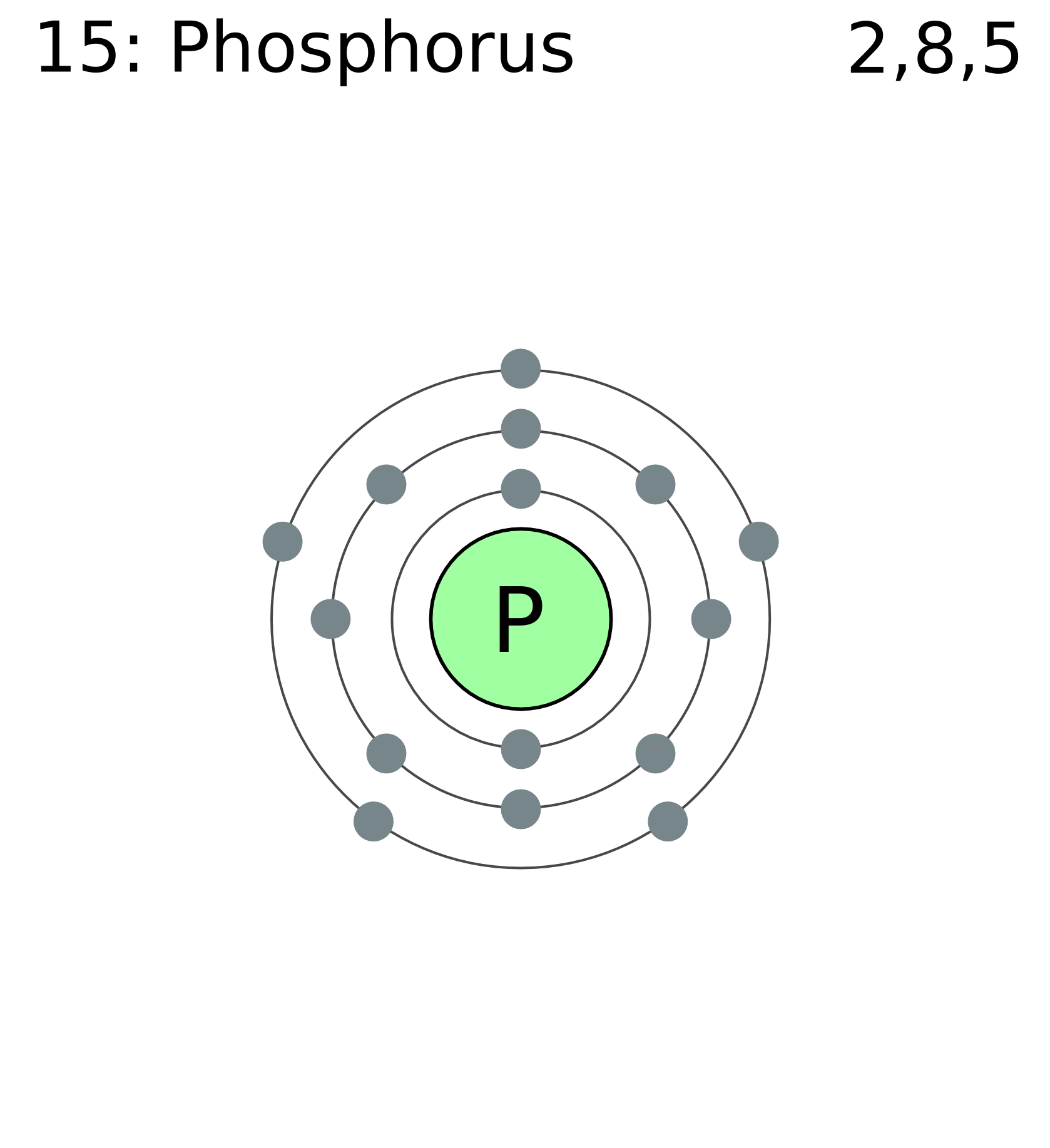
Phosphorus Electron Configuration (P) with Orbital Diagram
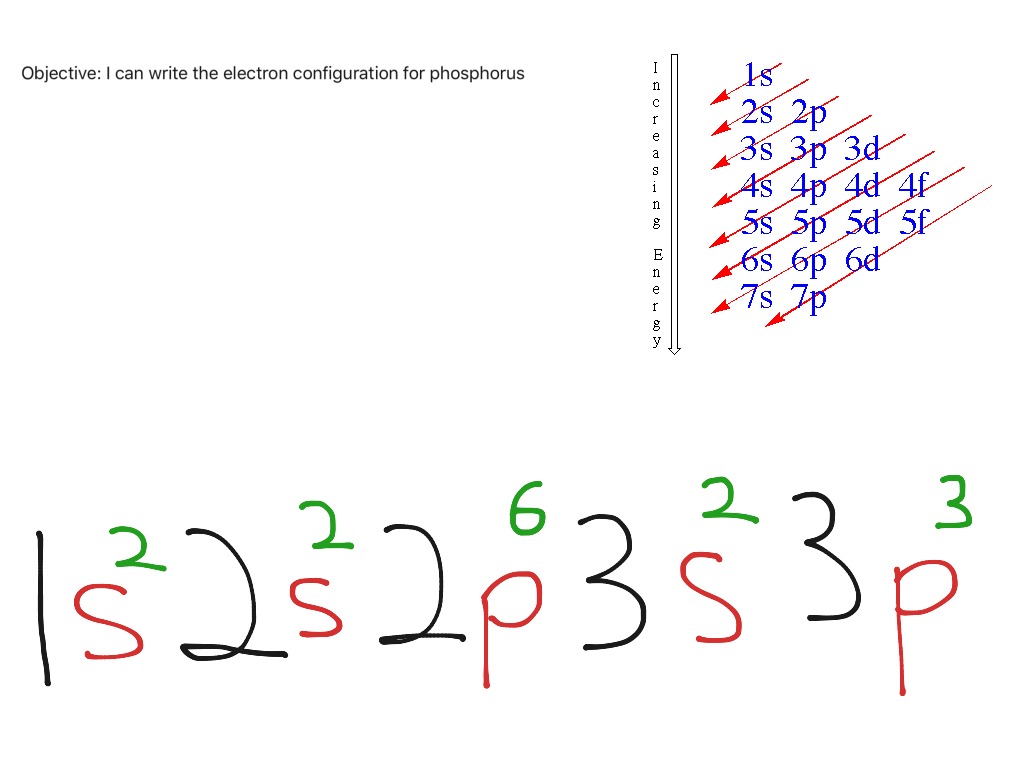
Electron configuration example for phosphorus Science, Chemistry
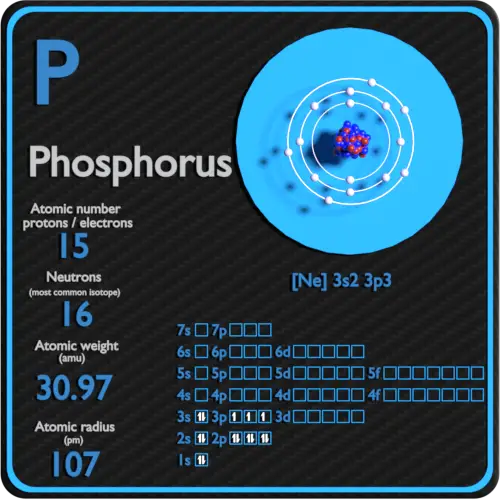
Phosphorus Protons Neutrons Electrons Electron Configuration
First Ionisation Energy The Minimum Energy Required To Remove An Electron From A Neutral Atom In Its.
O Electronic Structure Drawing A Box Diagram Of The Electron Configuration Of An Atom Draw The Electron Configuration For A Neutral Atom Of Phosphorus.
The Upper Right Side Shows The Number Of Electrons In A Neutral Atom.
Two Electrons Can Go Into The 1 S Subshell, 2 Can Go Into The 2 S Subshell, And 6 Can Go Into The 2 P Subshell.
Related Post: