Draw The Electron Configuration For A Neutral Atom Of Scandium
Draw The Electron Configuration For A Neutral Atom Of Scandium - Draw the electron configuration for a neutral atom of boron. Web now, the electron configuration of an atom can be built by filling the electrons in a lower energy subshell first then higher, higher, and higher. 83% (24 ratings) transcribed image text: O electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of scandium. The valence orbitals may have different ordering (i.e. Web electrons and electron configuration. Therefore, the number of electrons in neutral atom of scandium is 21. Draw the electron configuration for a neutral atom of scandium video answer: This turns out to be argon 4s 1, 3d 1 or once again you could write argon, 3d 1, 4s 1. This problem has been solved! The valence orbitals may have different ordering (i.e. Scandium atom is [ar] 3d1 4s2.the portion of scandium configuration that is equivalent to the noble gas of the preceding period, is. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) scandium atom (bohr model) Energy 3p click to change la. Scandium metal commonly forms sc^ (3+) ions. The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). (b) how many valence electrons are found in this element? The ground state abbreviated electronic configuration of neutral. Following hydrogen is the noble gas helium, which has an atomic number of 2. Scandium atom is [ar] 3d1 4s2.the portion. You will get the detailed information about the periodic table which will convert a newbie into pro. Energy 3p click to change la. Web so now let's think about what the electron configuration of the scandium would be. Draw the electron configuration for a neutral atom of scandium video answer: Scandium electron configuration using the aufbau principle a scandium atom. The atomic number of titanium is 22 and atomic number os scandiu. (b) how many valence electrons are found in this element? Web the electron configuration and the orbital diagram are: Draw the electron configuration for a neutral atom of boron. Therefore, the number of electrons in neutral atom of scandium is 21. Well scandium has one more proton than calcium. This turns out to be argon 4s 1, 3d 1 or once again you could write argon, 3d 1, 4s 1. Energy 3p click to change la. It has 21 protons and if it is neutral, it's also gonna have one more electron relative to a neutral calcium atom. Draw the electron. The electron configuration of a neutral atom of scandium is 1s2 2s2 2p6 3s2 3p6 4s2 3d1. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The valence orbitals may have different ordering (i.e. So some things that you need to note along the left side, these rows are numbered and those numbers. Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Draw the electron configuration for a neutral atom of boron. (a) draw and orbital diagram for this element. (b) how many valence electrons are found in this element?. Thus, it is simple to determine the charge on such a negative ion: Scandium atom is [ar] 3d1 4s2.the portion of scandium configuration that is equivalent to the noble gas of the preceding period, is. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. The valence orbitals may have different ordering. The first electron has the same four quantum numbers as the hydrogen atom electron (n= 1, l= 0, ml= 0, ms=+12ms=+12). (a) draw and orbital diagram for this element. Energy 3p click to change la. Make sure to label each sublevel and populate with the appropriate numbers of electrons. You can effortlessly find every single detail about the elements from. Scandium metal commonly forms sc^ (3+) ions. Web for example if you form the scandium plus one ion, the electron configuration for the scandium plus one ion, so we're losing an electron from a neutral scandium atom. Instead of having three electrons in the outer shell, scandium adds its electron to the second to last shell. The valence orbitals may. Web draw the electron configuration for a neutral atom of scandium. Well scandium has one more proton than calcium. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) scandium atom (bohr model) Web most monatomic anions form when a neutral nonmetal atom gains enough electrons to completely fill its outer s and p orbitals, thereby reaching the electron configuration of the next noble gas. Following hydrogen is the noble gas helium, which has an atomic number of 2. Draw the electron configuration for a neutral atom of scandium video answer: Web so now let's think about what the electron configuration of the scandium would be. Web the electron configuration of scandium is [ ar] 3d 1 4s 2 , if the electron arrangement is through orbitals. Electronegativity (pauling scale) the tendency of an atom to attract electrons towards itself, expressed on a relative scale. Web so, the scandium atom is neutral, hence, its number of electrons will be equal to the number of protons which is 21 as we already discussed. You can effortlessly find every single detail about the elements from this single interactive periodic table. Scandium electron configuration using the aufbau principle a scandium atom is a neutral atom that has an atomic number of 21 which implies it has a total of 21 electrons. The charge is equal to the number of electrons that must be gained to fill the s and p. Energy 3p click to change la. Web learn about the electron configuration diagram for scandium, a transition metal commonly found in minerals and used in various industrial applications. Pause this video and think about that.
Pin on Chemistry

Orbital Diagram For Scandium
:max_bytes(150000):strip_icc()/Scandium-58b6023e3df78cdcd83d49e1.jpg)
Atoms Diagrams Electron Configurations of Elements

Scandium Atom Science Notes and Projects
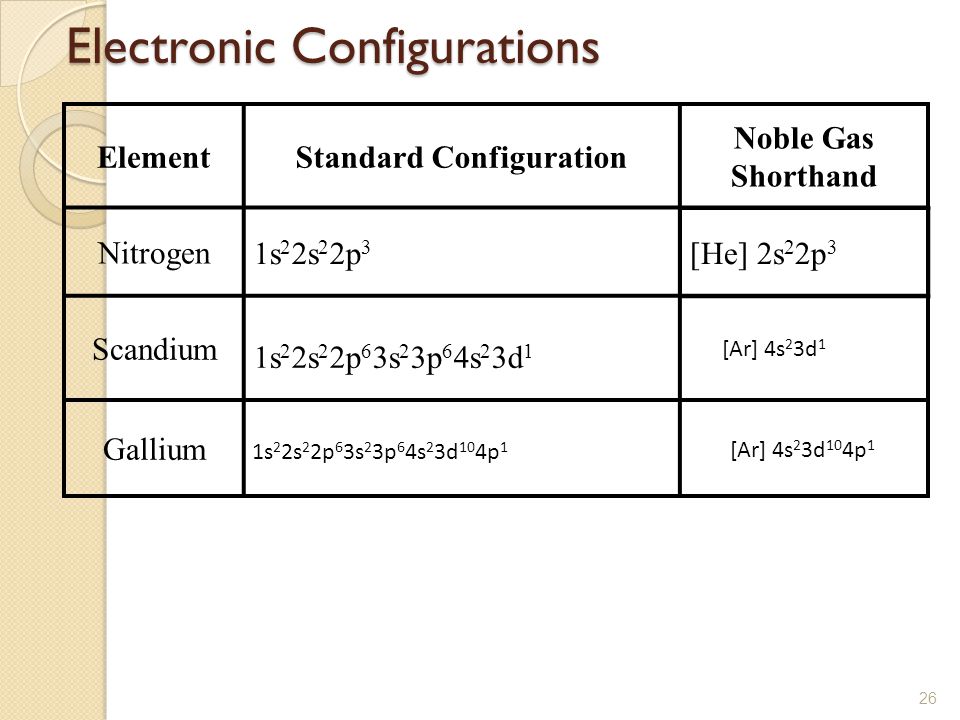
Scandium Electron Configuration (Sc) with Orbital Diagram
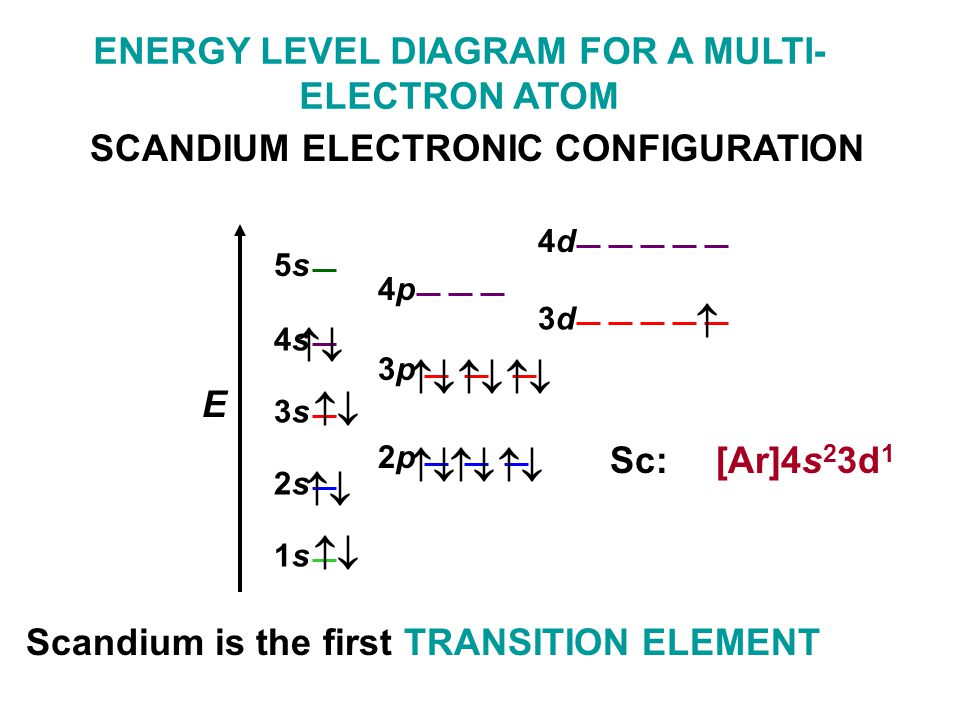
Scandium Electron Configuration (Sc) with Orbital Diagram
Scandium electron configuration Stock Image C029/5028 Science Photo
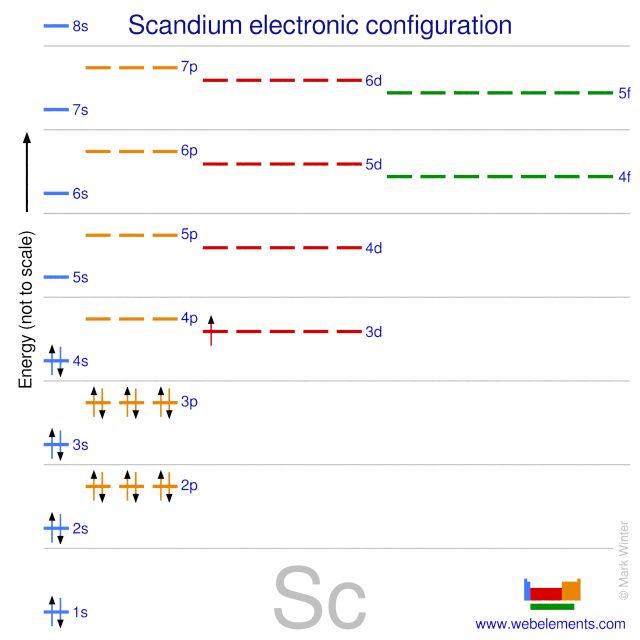
WebElements Periodic Table » Scandium » properties of free atoms
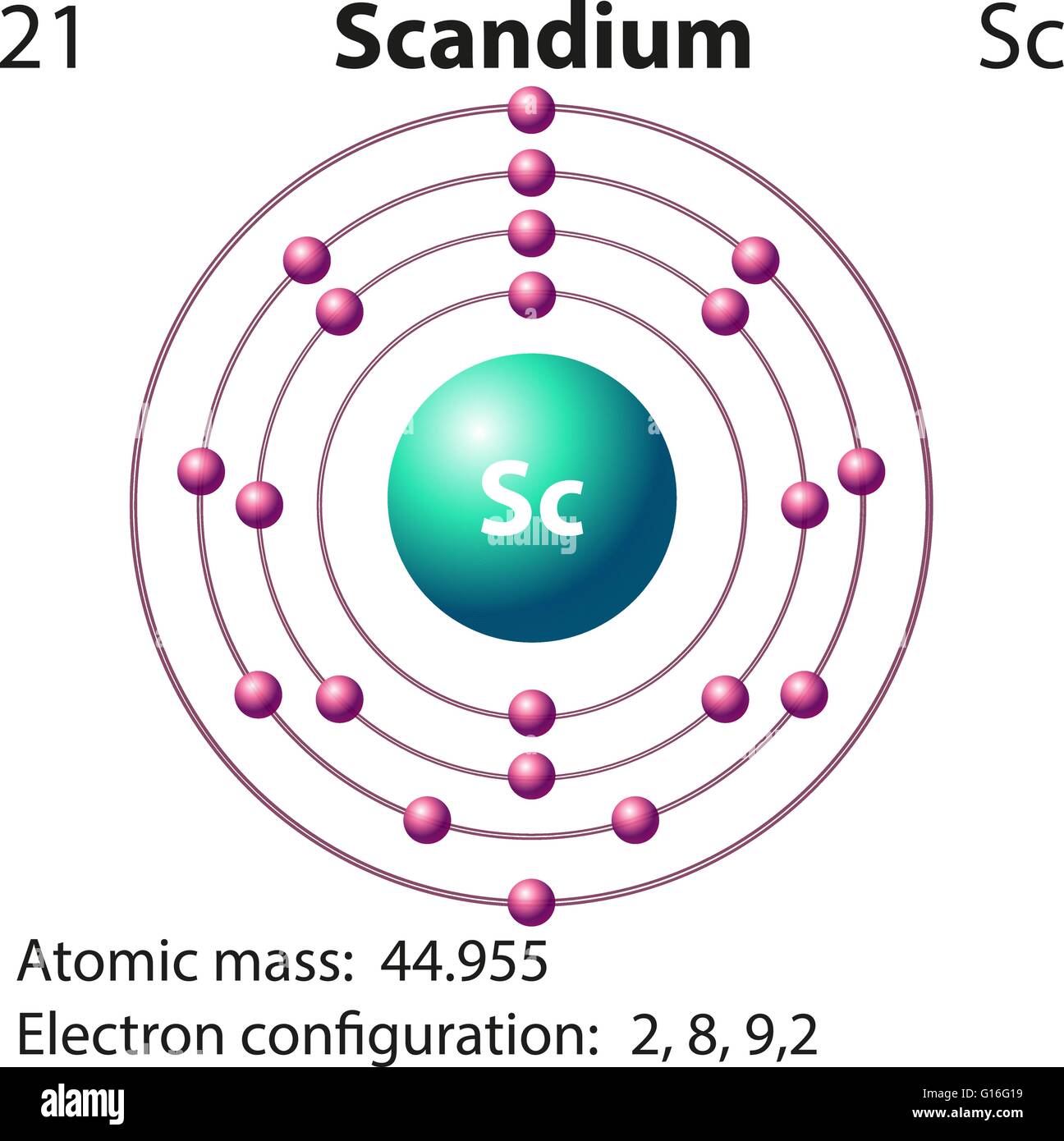
Symbol and electron diagram for Scandium illustration Stock Vector Art

Scandium electronic configuration How to Write Scandium electronic
First Ionisation Energy The Minimum Energy Required To Remove An Electron From A Neutral Atom In Its.
In Order To Write The Sc Electron Configuration We First Need To Kn.
Understand The Arrangement Of Electrons In Scandium's Energy Levels And Orbital Shells, Providing Insights Into Its Chemical Reactivity And Bonding Behavior.
The Helium Atom Contains Two Protons And Two Electrons.
Related Post:
