Draw The Lewis Structure For Ccl4
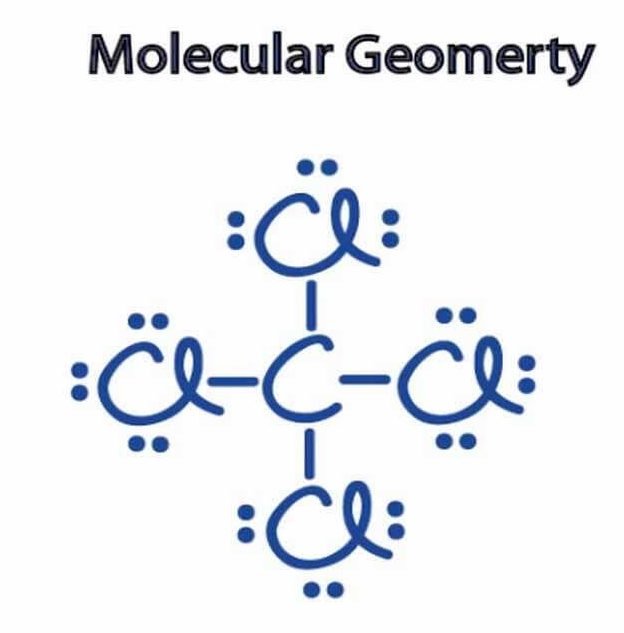
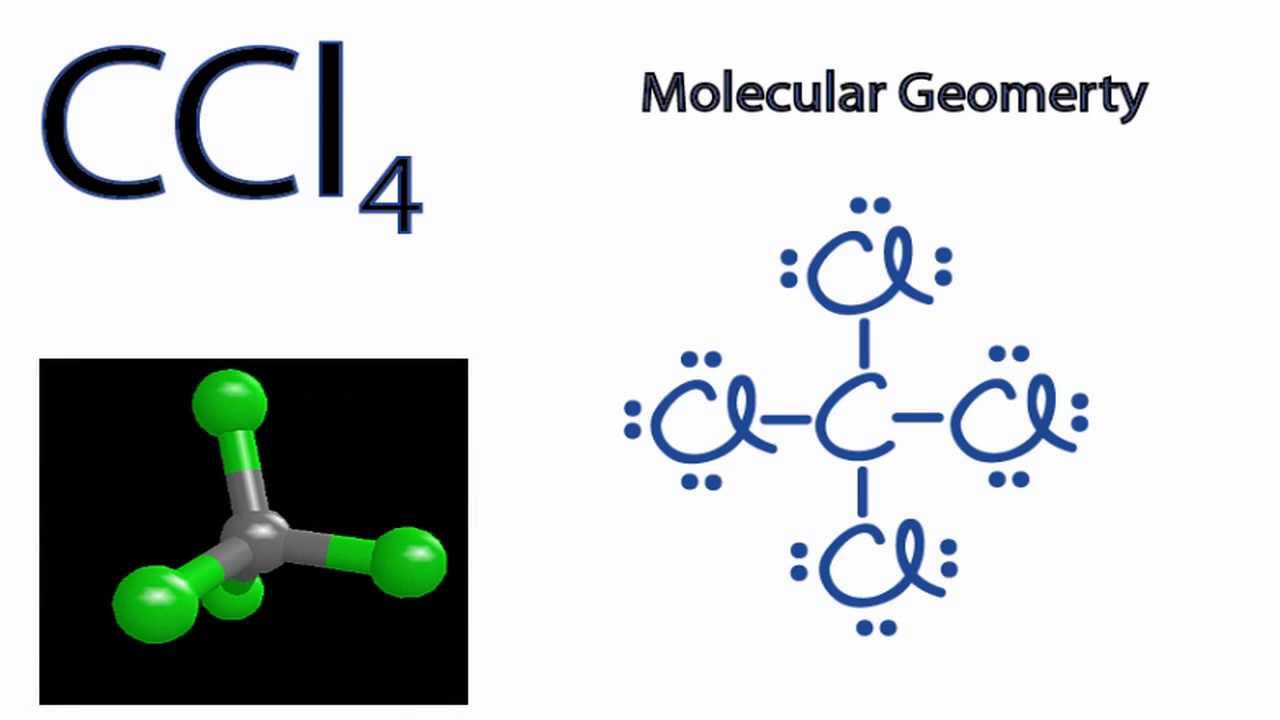
Draw The Lewis Structure For Ccl4 - Draw the lewis structure of ccl4. Web in the ccl 4 lewis structure, there are four single bonds around the carbon atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. Web 6 steps to draw the lewis structure of ccl4 step #1: While selecting the center atom, always put the least electronegative atom at the. Web try it free. Web the hybridization of ccl4 is sp3. Give the molecular shape around each carbon atom. How to draw a lewis structure for ccl4? We will learn how to draw the lewis structure of ccl 4 step by step in this tutorial. P с ору este it ii chemdoodle when the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: I also go over hybridization, shape and bond angle. The bond angle between the atoms is somewhere around 109 degrees. Is the molecule polar or nonpolar? I quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). Web science chemistry chemistry questions and answers draw the lewis structure of ccl4. Chlorine has 7 valence electrons, but we have 4 chlorines so let's multiply that by 4. The lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. Ccl_4 has a tetrahedral geometry with bond angles of 109.5 °. Web chemistry chemistry questions and answers draw the lewis structure for ccl4. Draw lewis structures for ccl4. I quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. This is all about the compound ccl4, its lewis structure, hybridization, molecular geometry, polarity, applications, and mo diagram. = 4 + (4*7) = 4 + 28 = 32 valence electrons How. Web in the ccl 4 lewis structure, there are four single bonds around the carbon atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. Web the hybridization of ccl4 is sp3. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Four. Ccl_4 has a tetrahedral geometry with bond angles of 109.5 °. Here’s the best way to solve it. Carbon atom is the center atom and each chlorine atom has 3 lone pairs. Include all the lone pairs. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Explain why the carbon atoms in the two molecules have different shapes. Web lewis dot structure for ccl4. Web chemistry chemistry questions and answers draw the lewis structure for ccl4. This helps us to understand the geometry of ccl4 which is tetrahedral. Draw the lewis structure for ccl4. Web 6 steps to draw the lewis structure of ccl4 step #1: This helps us to understand the geometry of ccl4 which is tetrahedral. Draw the lewis structure for ccl4. P с ору este it ii chemdoodle when the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Web for the lewis. This is all about the compound ccl4, its lewis structure, hybridization, molecular geometry, polarity, applications, and mo diagram. I quickly take you through how to draw the lewis structure of ccl4 (carbon tetrachloride). The bond angle between the atoms is somewhere around 109 degrees. Web drawing lewis structures for molecules with one central atom: As there are four molecules of. How to draw a lewis structure for ccl4? Ccl_4 has a tetrahedral geometry with bond angles of 109.5 °. P с ору este it ii chemdoodle when the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Web drawing lewis structures for molecules with one central atom: Here, the given molecule is. How to draw lewis structure and find electron and molecular geometries, and find bond angle of a compound draw lewis structure for ccl4 and determine electron geometry,. The lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. Here’s the best way to solve it. Perchloric acid (hcio4) (aq) + tetraphosphorus decaoxide (s) phosphoric acid.. It is a commonly used solventand was historically used as a fire extinguisher. Include all the lone pairs. Ccl_4 has a tetrahedral geometry with bond angles of 109.5 °. Web the lewis structure of ccl4, also known as carbon tetrachloride, is a representation of how the atoms are arranged in the molecule. On scratch paper, draw the lewis structure for ccl4 then, referring to the structure, fill in the blanks: The electrons are represented with the help of circular dots. This is all about the compound ccl4, its lewis structure, hybridization, molecular geometry, polarity, applications, and mo diagram. This problem has been solved! This helps us to understand the geometry of ccl4 which is tetrahedral. Here’s the best way to solve it. The lewis structure for ccl4 is a commonly tested lewis structures on general. Web for the lewis structure of ccl4 first, let’s calculate the total valence electrons. Draw lewis structures for ccl4 and c2cl4. Web draw the lewis structure for ccl4 in the window below and then answer the questions that follow. Perchloric acid (hcio4) (aq) + tetraphosphorus decaoxide (s) phosphoric acid. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.
Basic Lewis Structures CCl4 and CO2 YouTube
CCL4 Molecular Geometry, Lewis Structure, Hybridization, And Everything

Lista 94+ Foto Estructura De Lewis Del Dioxido De Carbono Lleno
Lewis Dot Diagram For Ccl4 Free Wiring Diagram

CCl4 Lewis Structure YouTube

CCl4 Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar

CCl4 Lewis Structure How to Draw the Dot Structure for CCl4 (Carbon

Formal charge on carbon atom of C Cl4 molecule = (4 0(8/2)) =0
Solved Draw the Lewis structure for CCl4 and answer the

CCl4 Molecular Geometry Science Education and Tutorials
> Lewis Structure Here Are The Steps That I Follow When Drawing A Lewis Structure.
How To Draw Lewis Structure And Find Electron And Molecular Geometries, And Find Bond Angle Of A Compound Draw Lewis Structure For Ccl4 And Determine Electron Geometry,.
I Also Go Over Hybridization, Shape And Bond Angle.
As There Are Four Molecules Of Chlorine, We Will Calculate The Number Of Valence Electrons Accordingly.
Related Post:
