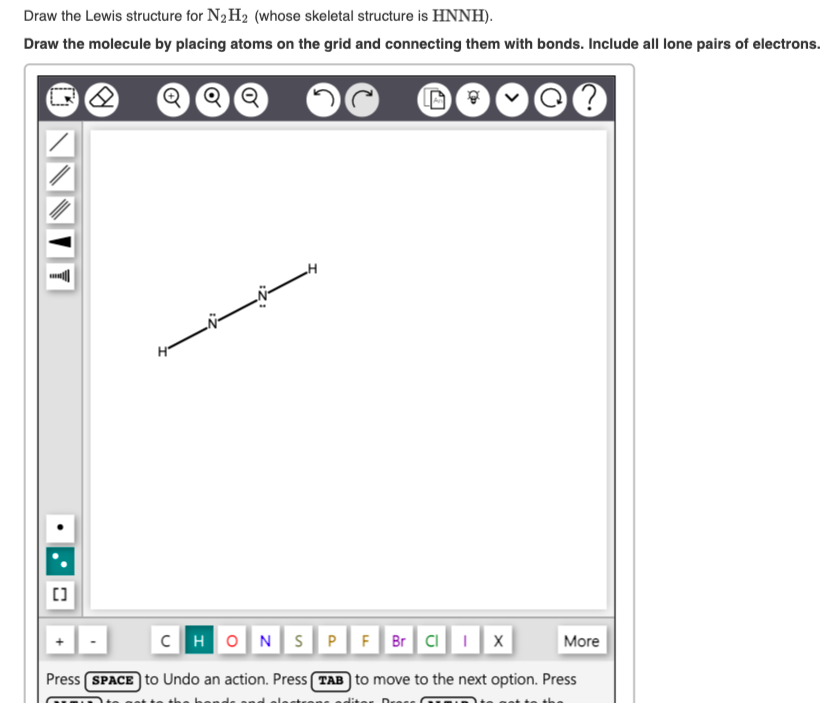
Draw The Lewis Structure For N2H2
Draw The Lewis Structure For N2H2 - Web chemistry questions and answers. Web the n2h2 lewis structure refers to the diagram that illustrates the bonding and electron distribution between nitrogen and hydrogen atoms in a molecule of dinitrogen hydride. The molecule has a chemical formula of n2h2 and to find out its lewis structure we first look at the total. Draw the lewis structures of n_2h_4, n_2h_2, and n_2. Understand the proper use of the octet rule to predict bonding in simple molecules. N 2 h 2 is straightforward with no double or triple bonds. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary #4 minimize formal charges by converting lone pairs of the atoms #5 repeat step 4 if necessary, until all charges are minimized Find the total valence electrons in n2h2 molecule in order to find the total valence electrons in n2h2 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. Draw the lewis structures of n2h4, n2h2, and n2. Web drawing the lewis structure for n 2 h 2. Let us find out the lewis structure of dinitrogen dihydride, n2h2. Draw the molecules by placing atoms on the grid and connecting them with bonds. In the n 2 h 2 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). Carbons 2 and 3 are sp3, carbons 4 and 5. The following. Verified solution 1m 620 6 2 mark as completed was this helpful? Let us find out the lewis structure of dinitrogen dihydride, n2h2. Hydrogen (h) only needs two valence electrons to have a full outer shell. Web write lewis symbols for neutral atoms and ions. Web lewis structure is a 2d diagrammatic representation of a molecular or an ionic structure. Find the total valence electrons in n2h2 molecule in order to find the total valence electrons in n2h2 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. Web chemistry questions and answers. Web today in this video we help you determine the lewis structure of dinitrogen dihydride. What is the. Web drawing the lewis structure for n 2 h 2. Find the total valence electrons in n2h2 molecule in order to find the total valence electrons in n2h2 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. • draw one structure per sketcher box, and separate added sketcher boxes with. Select the center atom (h is always outside). Web 6 steps to draw the lewis structure of n2h2 step #1: Web part a draw the lewis structure for n2h2. Web drawing lewis structures for molecules with one central atom: Web the lewis structure of n2h2 consists of two nitrogen (n) atoms at the center which are bonded to two atoms. Dinitrogen dihydride has the chemical formula of n2h2. Web the lewis structure of n2h2 consists of two nitrogen (n) atoms at the center which are bonded to two atoms of hydrogen (h), one on each side. • draw one structure per sketcher box, and separate added sketcher boxes with the + sign. It can be prepared from the decarboxylation of. Draw the lewis structures of n2h4, n2h2, and n2. Web determine the lewis dot structure for the diazene molecule, n 2 h 2. Web n2h2 lewis structure molecular geometry hybridization and mo diagram. Web watch on steps of drawing n2h2 lewis structure step 1: Web 6 steps to draw the lewis structure of n2h2 step #1: #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability let’s one by one discuss each step in detail. Verified solution 1m 620 6 2 mark as completed was this helpful? Draw the lewis structures of n2h4, n2h2, and n2. Draw lewis structures depicting the bonding. Web drawing lewis structures for molecules with one central atom: Include all lone pairs of electrons and hydrogen atoms. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability let’s one by one discuss each step in detail. Web part a draw the lewis structure for. Web by using the following steps, you can easily draw the lewis structure of n 2 h 2: The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web lewis structure is a 2d diagrammatic representation of a molecular or an ionic structure that helps us decipher the type. The molecule has a chemical formula of n2h2 and to find out its lewis structure we first look at the total. Hydrogen (h) only needs two valence electrons to have a full outer shell. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the molecules by placing atoms on the grid and connecting them with bonds. Here, the given molecule is n2h2 (or dinitrogen dihydride). Hch2 367 carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized. Include all lone pairs of electrons and hydrogen atoms. Web n2h2 lewis structure, molecular geometry, hybridization, bond angle and shape. Dinitrogen dihydride has the chemical formula of n2h2. #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 complete octet on central atom #5 calculate formal charge and check stability let’s one by one discuss each step in detail. In the n 2 h 2 lewis structure the two nitrogen (n) atoms go in the center (hydrogen always goes on the outside). A) ccl4 b) n2h2 c) hcn 6) a) draw the molecular orbital diagram of the given compounds. Web 6 steps to draw the lewis structure of n2h2 step #1: It can be prepared from the decarboxylation of azodicarboxylic acid 2 ). Web chemistry questions and answers. Carbons 2 and 3 are sp3, carbons 4 and 5.
How to Draw the Lewis Dot Structure for N2H2 Diazene YouTube

Draw the Lewis structures of N2H4, N2H2, and N2 YouTube

Lewis Dot Structure For N2h2 Draw Easy
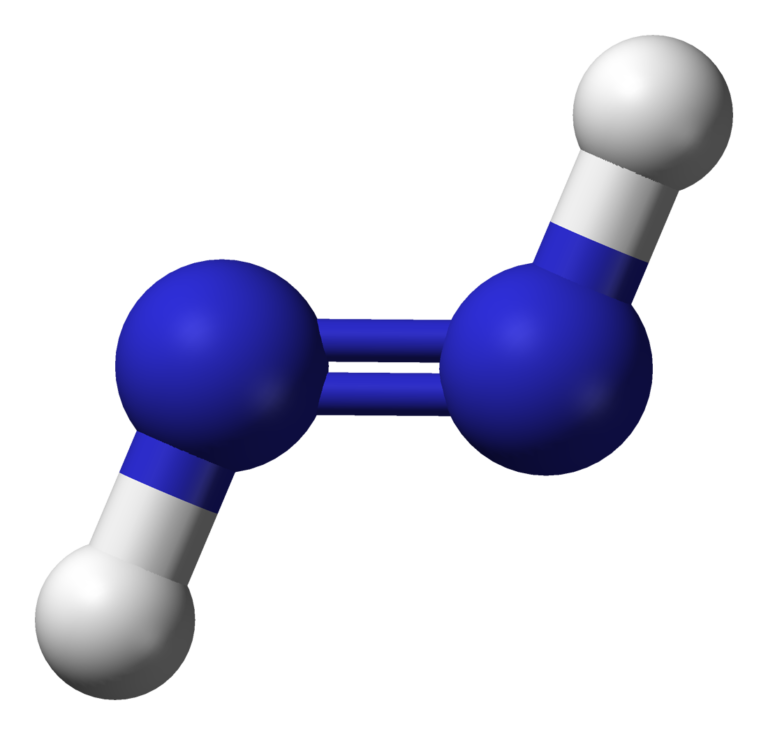
N2H2 Lewis structure, Molecular Geometry, Hybridization, Bond Angle and
Solved Draw the Lewis structure for N2H2 (whose skeletal

N2h2 Lewis Structure Molecular Geometry Draw Easy

N2H2 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube

N2H2 Lewis Structure (Dinitrogen Dihydride) How to find out
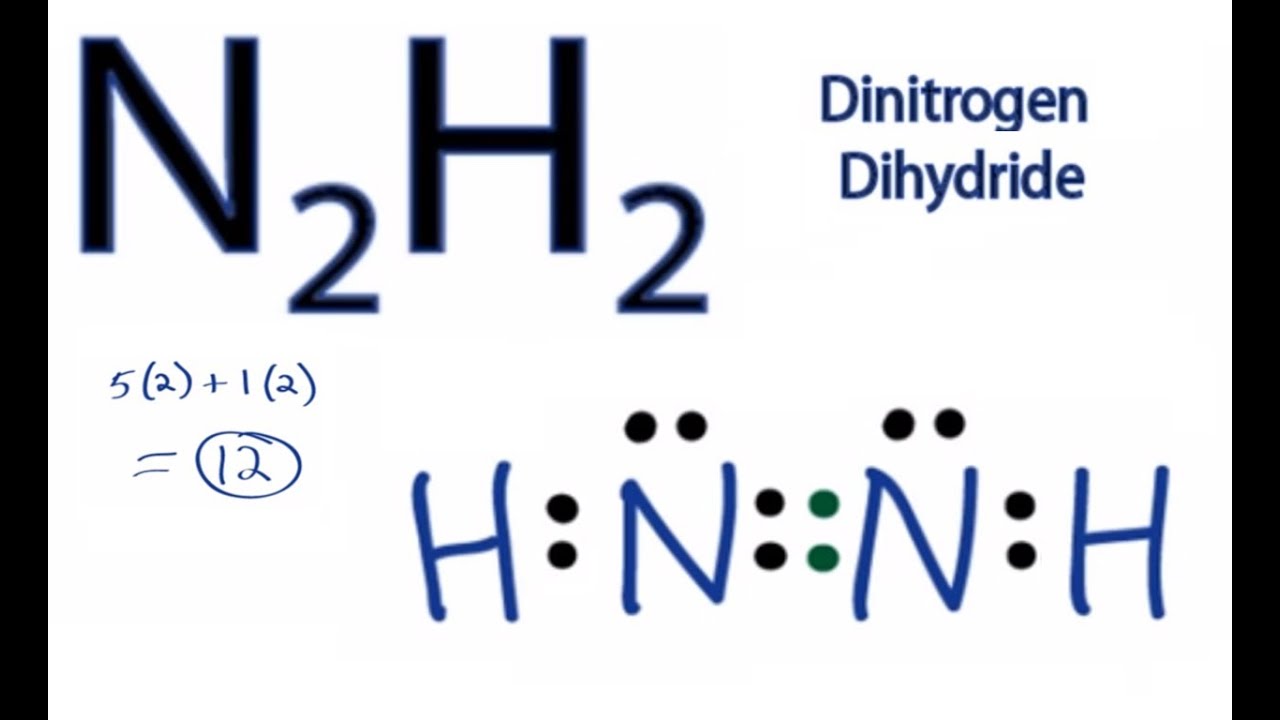
N2H2 Lewis Structure How to Draw the Lewis Structure for Dinitrogen
N2h2 Lewis Structure Molecular Geometry Draw Easy
To Draw The N2H2 Lewis Structure, The Valence Electrons Of Each Atom Are Represented By Dots Or Lines, While The Bonding Pairs Are Depicted By Lines Between The Atoms.
Verified Solution 1M 620 6 2 Mark As Completed Was This Helpful?
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
Web The N2H2 Lewis Structure Refers To The Diagram That Illustrates The Bonding And Electron Distribution Between Nitrogen And Hydrogen Atoms In A Molecule Of Dinitrogen Hydride.
Related Post: