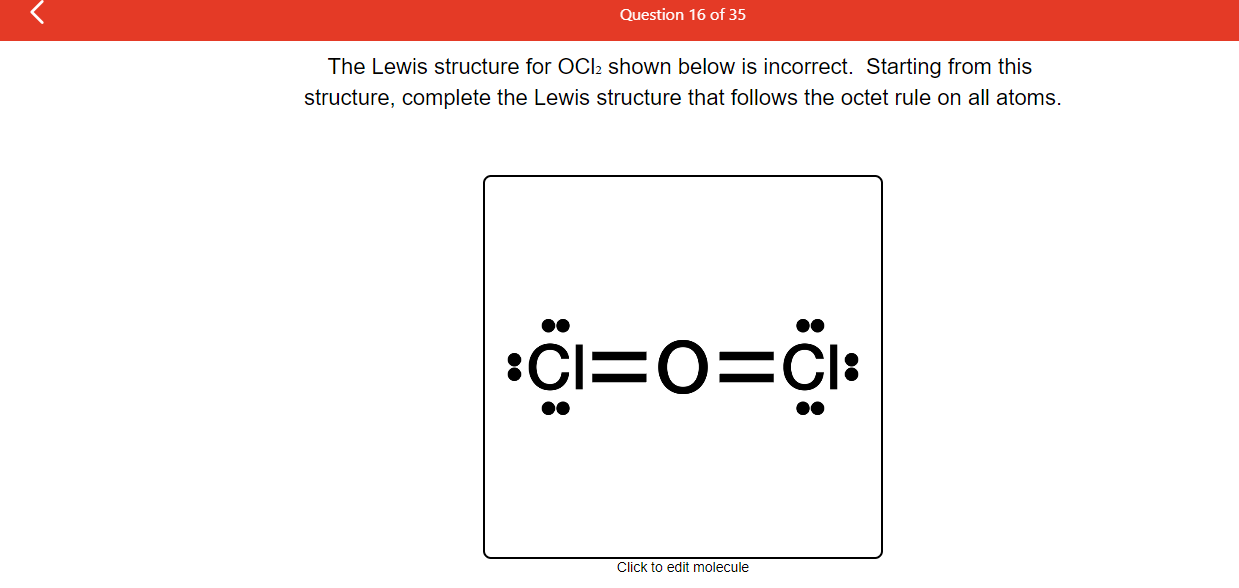
Draw The Lewis Structure For Nh2Oh
Draw The Lewis Structure For Nh2Oh - 5 + 6 + 2 = 13 selection of central atom to determine the central atom in nh2oh, we need. Web steps to properly draw the nh 2 oh lewis structure, follow these steps: There are a total of 14 valence electrons in the nh 2 oh lewis structure. Determine the number of valence… | bartleby. In order to draw the lewis. A) 16 b) 14 c) 13 d) 10 e) 15 click to edit molecule. This problem has been solved! Your answer choice is independent of the orientation of your drawn structure. Here, the given molecule is nh2oh. Web chemistry chemistry questions and answers draw the lewis structure for nh,oh. Science chemistry determine the number of valence electrons in nh2oh and then draw the corresponding lewis structure. Web chemistry chemistry questions and answers draw the lewis structure for nh,oh. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. A) 16 b) 14 c) 13 d) 10. It is a chemical formula for hydroxylamine. Also, helium is shown in group 8a, but it only has two valence electrons. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web steps to properly draw the nh 2 oh lewis structure, follow these steps: 6 valence electrons hydrogen (h): A) 16 b) 14 c) 13 d) 10 e) 15 click to edit molecule. Also, helium is shown in group 8a, but it only has two valence electrons. Web the lewis structure of nh2oh is made up of one nitrogen (n), three hydrogens (h), and one. Calculate the formal charge of central atom. This problem has been solved! In nh2cooh, we have nitrogen (n), carbon (c), and two oxygen (o) atoms. Web can someone explain step by step how to draw the lewis structure for nh2oh this problem has been solved! Web the first step in drawing the lewis structure of nh2cooh is to determine the total number of valence electrons involved. Find. A) 16 b) 14 c) 13 d) 10 e) 15 click to edit molecule. Calculate the formal charge of central atom. Calculate the total number of valence electrons. Also, helium is shown in group 8a, but it only has two valence electrons. Web the first step in drawing the lewis structure of nh2cooh is to determine the total number of. Determine the energy of a photon of each of these wavelengths. Calculate the formal charge of central atom. Web the first step in drawing the lewis structure of nh2cooh is to determine the total number of valence electrons involved. Science chemistry question nh2oh lewis structure solution verified answered 1 year ago Nitrogen (n) goes at the center of the nh. The lewis structure is a simplified representation of the valance shell electrons… You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web draw a lewis structure for each of the following:a. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in. Determine the number of valence electrons in nh2oh and then draw the corresponding lewis structure. Web the nh2o lewis structure refers to the arrangement of atoms and electrons in a molecule of nh2o. Web drawing the lewis structure for nh 2 oh. Web nh2oh lewis structure | quizlet related questions with answers the red line in the emission spectrum of. Draw the molecule by placing atoms on the grid and connecting them with bonds. Determine the number of valence electrons in nh2oh and then draw the corresponding lewis structure. Select the center atom (h is always outside). Calculate the formal charge of central atom. Web hello everyone welcome back to our channel, and in today's video, we will help you. Here, the given molecule is nh2oh. Valence electrons are the outermost electrons of an atom that participate in bonding. A) 16 b) 14 c) 13 d) 10 e) 15 click to edit molecule. C2br2draw a lewis structure , including the resonance forms, for each of the following molecules or ions.a. Science chemistry question nh2oh lewis structure solution verified answered 1. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. The lewis structure is a simplified representation of the valance shell electrons… Determine the number of valence… | bartleby. 5 + 6 + 2 = 13 selection of central atom to determine the central atom in nh2oh, we need. This problem has been solved! 2 valence electrons (2 x 1) total valence electrons in nh2oh: Also, helium is shown in group 8a, but it only has two valence electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web draw a lewis structure for each of the following:a. Calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Determine the number of valence electrons in nh2oh and then draw the corresponding lewis structure. 5 valence electrons oxygen (o): A) 16 b) 14 c) 13 d) 10 e) 15 click to edit molecule. There are a total of 14 valence electrons in the nh 2 oh lewis structure. While selecting the center atom, always put the least. Determine the energy of a photon of each of these wavelengths.
steps for drawing a lewis structure swansonmcarthurphysicaltherapy

Draw the Lewis structure for each of the following a BF4 b Cl2O c

Draw The Lewis Structure
[Solved] Draw a Lewis structure for each of the following a. NH2OH b
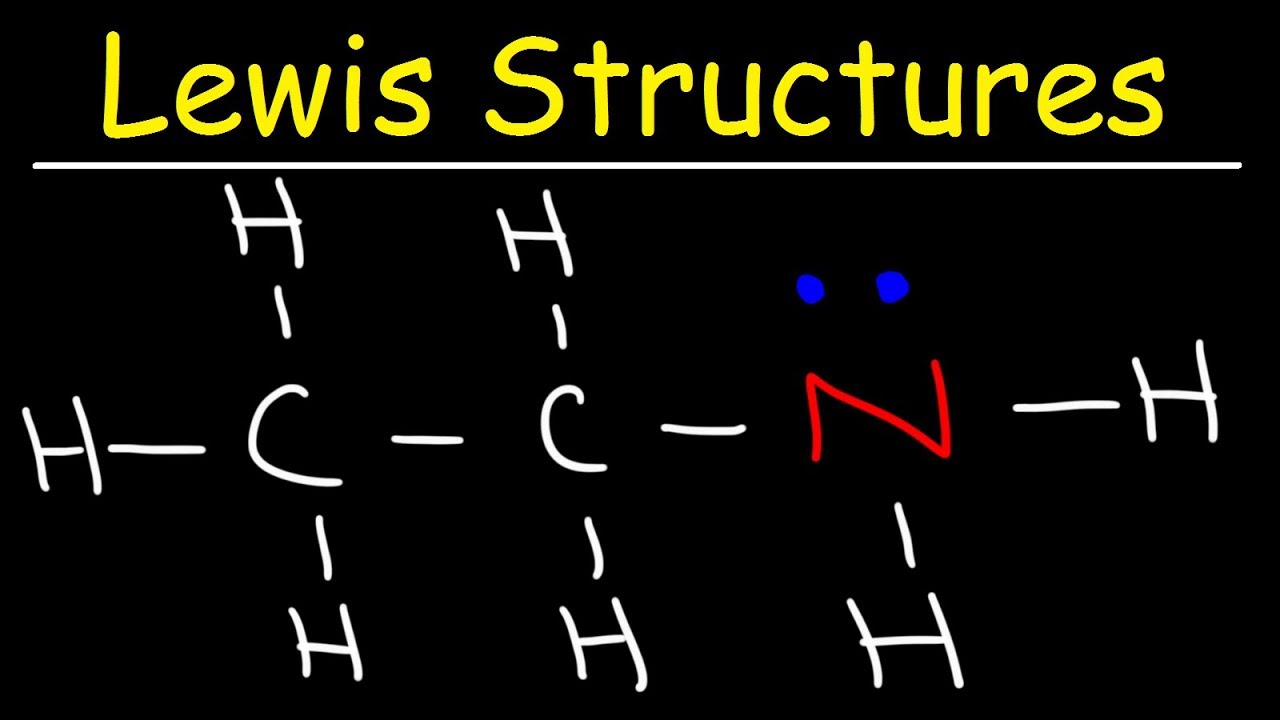
Organic Chemistry How To Draw Lewis Structures YouTube

1 Draw a Lewis structure for each of the following a NH2OH b C2H3Cl c

NH2OH lewis structure, Molecular geometry, and Bond angle
Solved Determine the number of valence electrons in NH2OH

NH2OH Lewis structure, Molecular geometry, Hybridization, Polar or

NH2OH Lewis Structure How to Draw the Lewis Structure for NH2OH YouTube
Web Draw The Lewis Structure Of Nh2Oh And Then Choose The Appropriate Pair Of Molecular Geometries Of The Two Central Atoms.
Valence Electrons Are The Outermost Electrons Of An Atom That Participate In Bonding.
Web The First Step In Drawing The Lewis Structure Of Nh2Cooh Is To Determine The Total Number Of Valence Electrons Involved.
Web By Using The Following Steps, You Can Easily Draw The Lewis Structure Of Nh 2 Oh.
Related Post: