Draw The Lewis Structure For Pf3 Including Lone Pairs
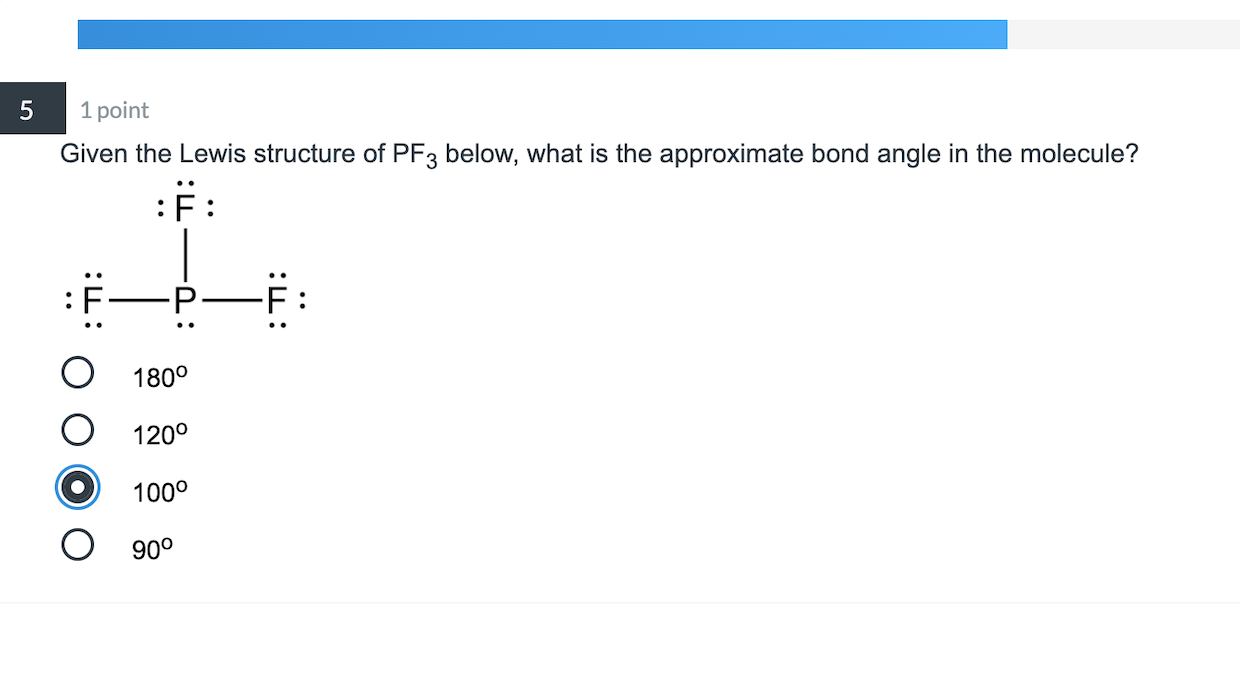
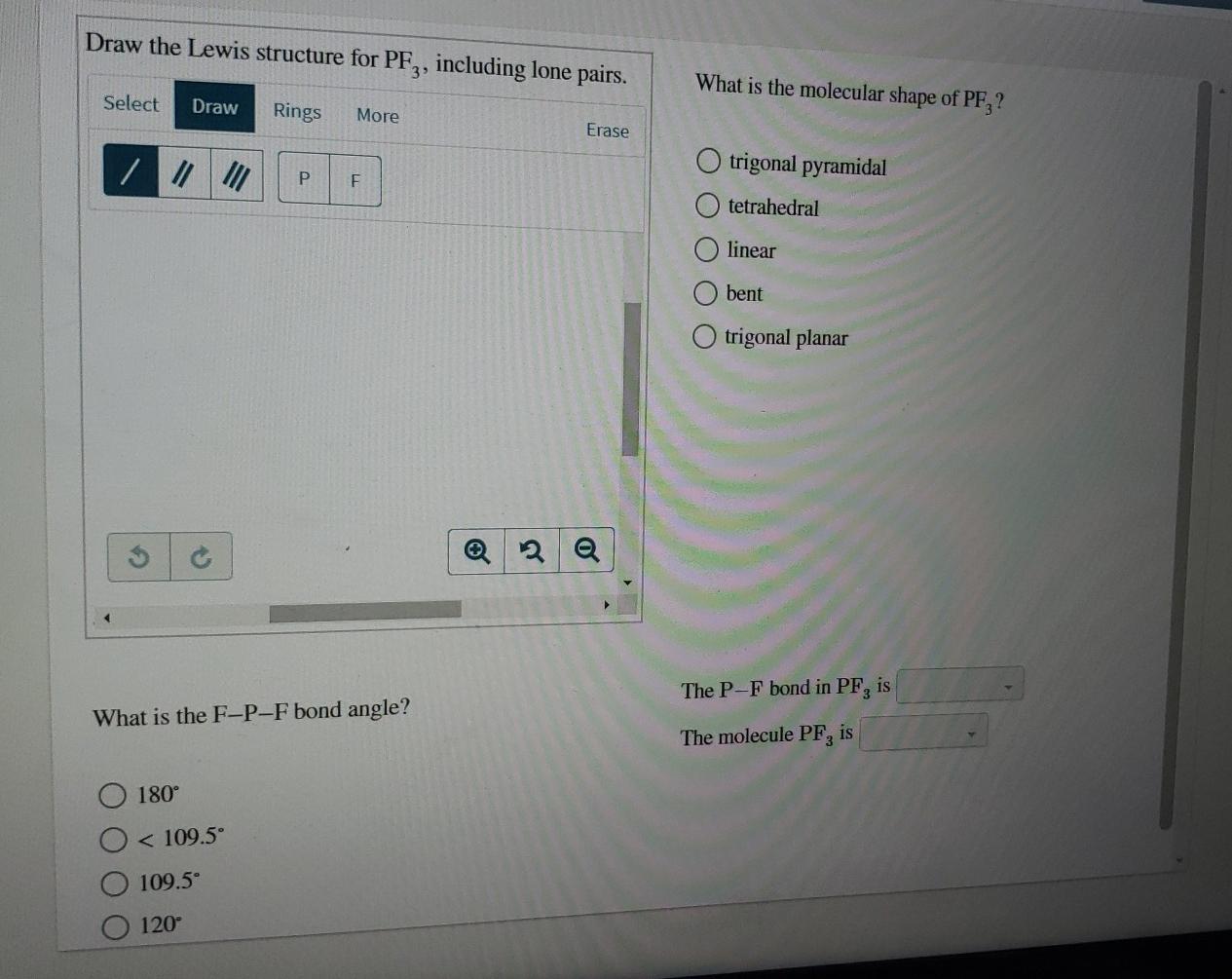
Draw The Lewis Structure For Pf3 Including Lone Pairs - It means the molecule will produce four new hybrid orbitals of. Web chemistry chemistry questions and answers draw a lewis structure for each of the following compounds. Pf3 draw the lewis structure for pf3 in the box at the right, including lone pairs. This problem has been solved! Count total valence electron in pf3 in the first step, we have to find how many valence electrons are there in pf3, so that we can distribute them around central and terminal atoms with the goal of completing their octet shell. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web pof3 lewis structure lone pairs. Include all lone pairs of electrons and nonbonding electrons. Web we show you how to draw the lewis structure and determine the molecular geometry for phosphorus trifluoride (pf3). Web chemistry chemistry questions and answers draw a lewis structure for each of the following molecules. A b oneclass 13.7k subscribers subscribe 207 views 2 years ago 🚀to book a personalized. Each chlorine is a halogen. Here, the given molecule is pf3 (phosphorus trifluoride). Write a lewis structure for the phosphorus trifluoride molecule, pf3. Pf 3 is a polar molecule. How many lone pairs are there on the phosphorus atom loading see answer loading plus add answer+10 pts loading ask ai more log in to add comment Calculate the total number of valence electrons. So each chlorine has seven valence electrons. The lewis structure has chloral ethane. Using formal charges to determine how many bonds to make, a different perspective. It means the molecule will produce four new hybrid orbitals of. Pf 3 is a neutral compound so no charge appears on this compound. Web the first step is to sketch the lewis structure of the pf3 molecule, to add valence electrons around the phosphorus atom; Include all lone pair electrons. The lewis structure of pf3 contains three single bonds,. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary let’s break down each step in more detail. In the lewis structure of pof3, the central phosphorus atom has one lone pair of electrons. Web science chemistry chemistry questions and answers draw a lewis structure. Pf3 draw the lewis structure for pf3 in the box at the right, including lone pairs. Breaking the octet rule ; Include all lone pairs of electrons. Web chemistry chemistry questions and answers draw a lewis structure for each of the following molecules. The lewis structure has chloral ethane. Include all lone pairs of electrons. Count total valence electron in pf3 in the first step, we have to find how many valence electrons are there in pf3, so that we can distribute them around central and terminal atoms with the goal of completing their octet shell. Drawing lewis structures for bf3, pf3 and brf3; #1 draw a rough sketch. Web to properly draw the pf 3 lewis structure, follow these steps: Web we show you how to draw the lewis structure and determine the molecular geometry for phosphorus trifluoride (pf3). Include all lone pair electrons. Alright, so let's go ahead and sketch that out where you're going to put grooming in the center surrested by the four florence. So. In the lewis structure of pof3, the central phosphorus atom has one lone pair of electrons. Pf3 draw the lewis structure for pf3 in the box at the right, including lone pairs. This problem has been solved! Pf3 c2f4 sbh3 this problem has been solved! Drawing lewis structures for bf3, pf3 and brf3; A b oneclass 13.7k subscribers subscribe 207 views 2 years ago 🚀to book a personalized. Web lewis structure of pf 3. Include all lone pairs of electrons. Web 05/02/2023 chemistry high school verified answered • expert verified draw a lewis structure for pf3. What is the molecular shape of bf3? Include all lone pairs of electrons. For the pf3 structure use the periodic table to find the total number of valence. Web we show you how to draw the lewis structure and determine the molecular geometry for phosphorus trifluoride (pf3). Alright, so let's go ahead and sketch that out where you're going to put grooming in the center surrested by. Include all lone pairs of electrons. Count total valence electron in pf3 in the first step, we have to find how many valence electrons are there in pf3, so that we can distribute them around central and terminal atoms with the goal of completing their octet shell. Pf 3 is a neutral compound so no charge appears on this compound. Bf3 draw the lewis structure for bf3 in the box at the right, including lone pairs. Web simple steps for drawing the lewis dot structure for pf3 1. Web chemistry chemistry questions and answers draw a lewis structure for each of the following molecules. Include all lone pair electrons. It means the molecule will produce four new hybrid orbitals of. Web as a single phosphorus trifluoride (pf3) molecule has three bonds (between phosphorus and fluorine) and one lone pair of electrons, the steric number is four. For the pf3 structure use the periodic table to find the total number of valence. Include all lone pairs of electrons. Web to properly draw the pf 3 lewis structure, follow these steps: A b oneclass 13.7k subscribers subscribe 207 views 2 years ago 🚀to book a personalized. The lewis structure of pf3 contains three single bonds, with phosphorus in the center, and three fluorines on either side. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. We have to count the total number of electrons.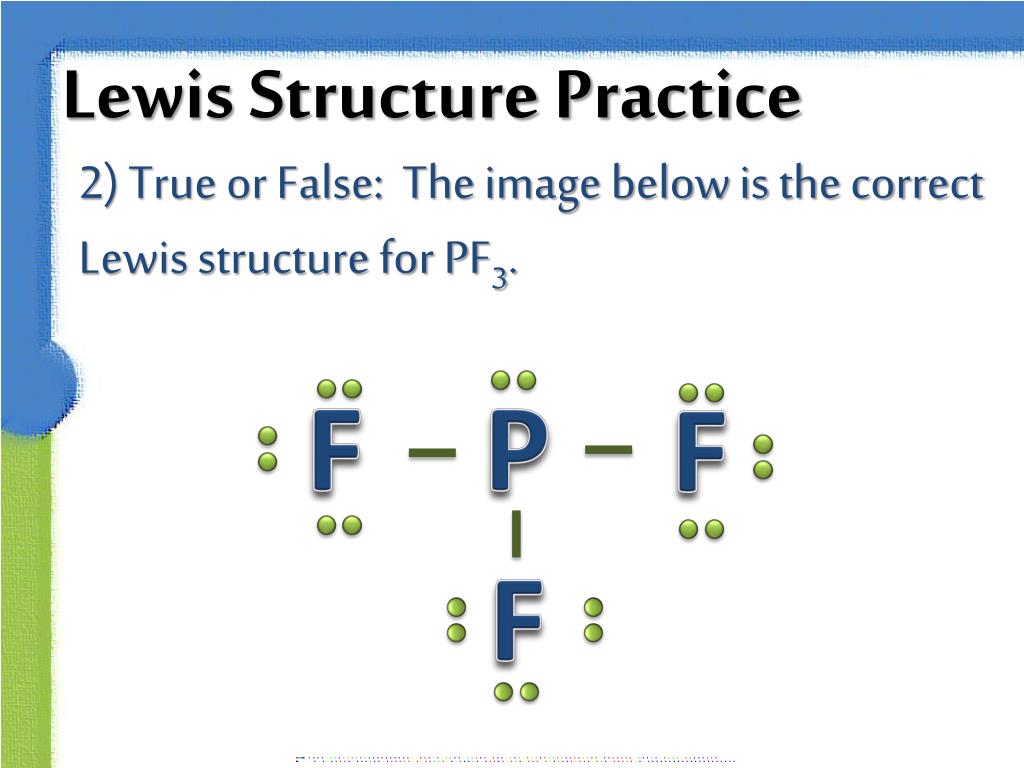
PPT Covalent Bonding Lewis Structures PowerPoint Presentation, free

draw the lewis structure for pf3 including lone pairs

PF3 Molecular Geometry Science Education and Tutorials

draw the lewis structure for pf3 including lone pairs 160vanbruntstreet
Lewis Structure For Pf3 Drawing Easy

The first step is to put five valence electrons around the phosphorus

Pf3 Lewis Structure How To Draw The Lewis Structure For
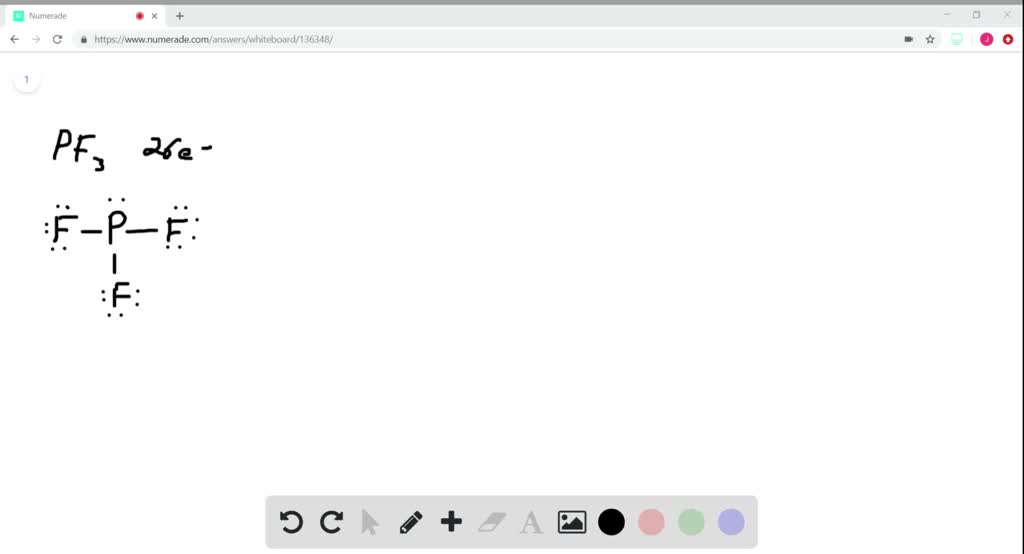
SOLVED(a) Draw the dominant Lewis structure for the phosphorus
draw the lewis structure for pf3 including lone pairs bettinaniedermaier

42+ Pf3 Electron And Molecular Geometry Full GM
Calculate The Total Number Of Valence Electrons.
Breaking The Octet Rule ;
#1 Draw A Rough Sketch Of The Structure First, Determine The Total Number Of Valence Electrons
Each Chlorine Is A Halogen.
Related Post: