Draw The Lewis Structure For Sih4
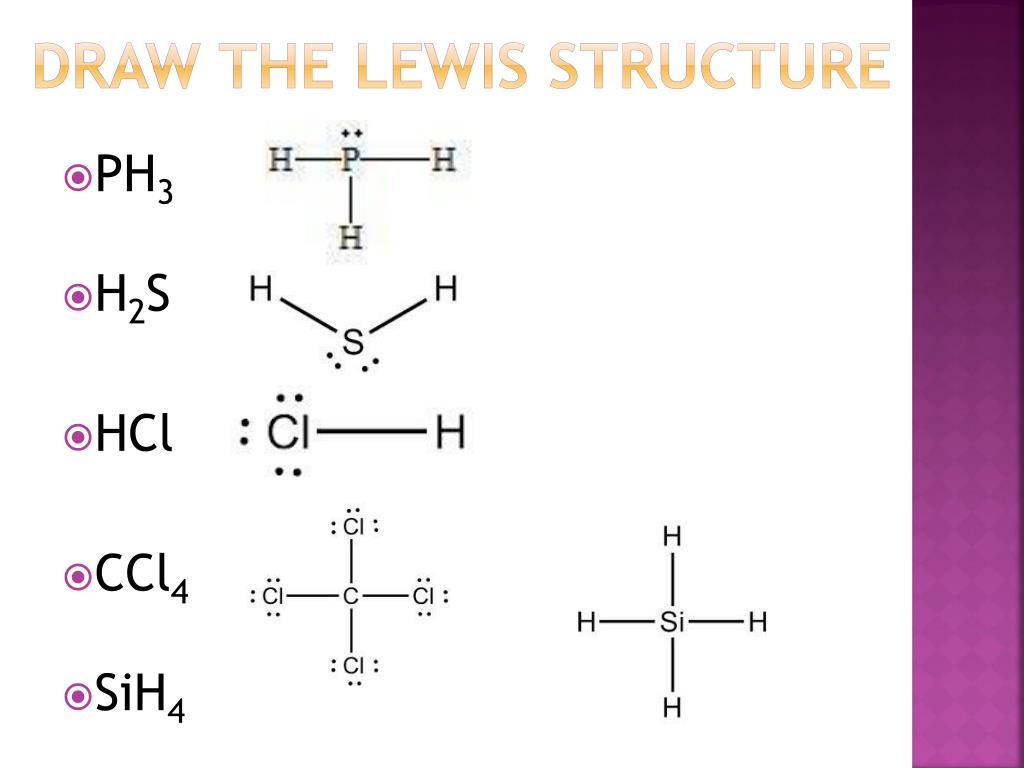
Draw The Lewis Structure For Sih4 - Start with the silicon atom in the center and draw four single bonds to the hydrogen atoms. The sum of the valence electrons is. Draw the lewis structure for each molecule. Include lone pairs, as needed. Determine the total number of valence (outer shell) electrons. A draw the lewis structure for sih4 in the window below and then decide if the molecule is polar or nonpolar. Draw the lewis structure for this compound. Draw the lewis structure of sih4. For the sih4 structure use the periodic table to find the total number of valence. For the sih4 structure use the periodic table to find the total number of valence electron.more. Sih4 is also called silane or monosilane, it is a colorless flammable and poisonous gas with a strong pungent odor. Include lone pairs, as needed. Each hydrogen atom should have two electrons around it, one from the bond and one as a lone pair. #1 draw a rough sketch of the structure first, determine the total number of valence electrons. #1 draw skeleton #2 show chemical bond #3 calculate formal charge and check stability (if there are no lone pairs and octet is already completed on central atom) let’s one by one discuss each step in detail. In order to draw the lewis. For the compound sih4, a. Web chemistry chemistry questions and answers draw the lewis structure of sih4.. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Include all lone pairs of electrons. This problem has been solved! Web the lewis structure for sih 4 has 8 valence electrons available to work with. You'll get a detailed solution from a subject matter expert that helps you learn core. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Sha s i h a. Draw the lewis structure for each molecule. Welcome back to our channel, and in today’s video, we will help you do sih4 lewis structure. The following procedure will give you the correct lewis structure for any. Okay, so you asked to draw the loose structure of i h four. Include lone pairs, as needed. Draw the lewis symbol for each element in the molecule. Find the total valence electrons in sih4. Predict the geometry of this compound. Find the total valence electrons in sih4. Web the lewis structure for sih 4 has 8 valence electrons available to work with. Select the center atom (h is always outside). Web this problem has been solved! Web to draw the lewis structure for sih4, follow these steps: Follow this video to know the detailed method an. Web 6 steps to draw the lewis structure of sih4 step #1: Predict the geometry of this compound. Web the lewis structure for sih 4 has 8 valence electrons available to work with. Is this compound ionic, polar covalent, or nonpolar covalent? Select the center atom (h is always outside). Is this compound ionic, polar covalent, or nonpolar covalent? Find the total valence electrons in sih4. In this article,”sih4 lewis structure”, different facts like lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. Predict the geometry of this compound. Sha s i h a. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure for each molecule. Calculate the total number of valence electrons. Draw the molecule by placing atoms on the grid and connecting them with bonds. Draw the lewis structure for co. Draw the lewis symbol for each element in the molecule. Web chemistry chemistry questions and answers draw the lewis structure of sih4. Draw a lewis structure for silane (sih4) and predict its molecular geometry. While selecting the center atom, always put the least. For the sih4 structure use the periodic table to find the total number of valence electron.more. In this article,”sih4 lewis structure”, different facts like lewis structure drawing, formal charge calculation, hybridization, structure with some detailed explanations are described below. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Please show the steps you went through to obtain your final structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Follow this video to know the detailed method an. The sum of the valence electrons is. Count the number of electrons around each atom. Draw the lewis structure for this compound. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Welcome back to our channel, and in today’s video, we will help you do sih4 lewis structure. Include all lone pairs of electrons. Draw the lewis structure for each molecule. Calculate the total number of valence electrons. Select the center atom (h is always outside). #1 draw a rough sketch of the structure first, determine the total number of valence electrons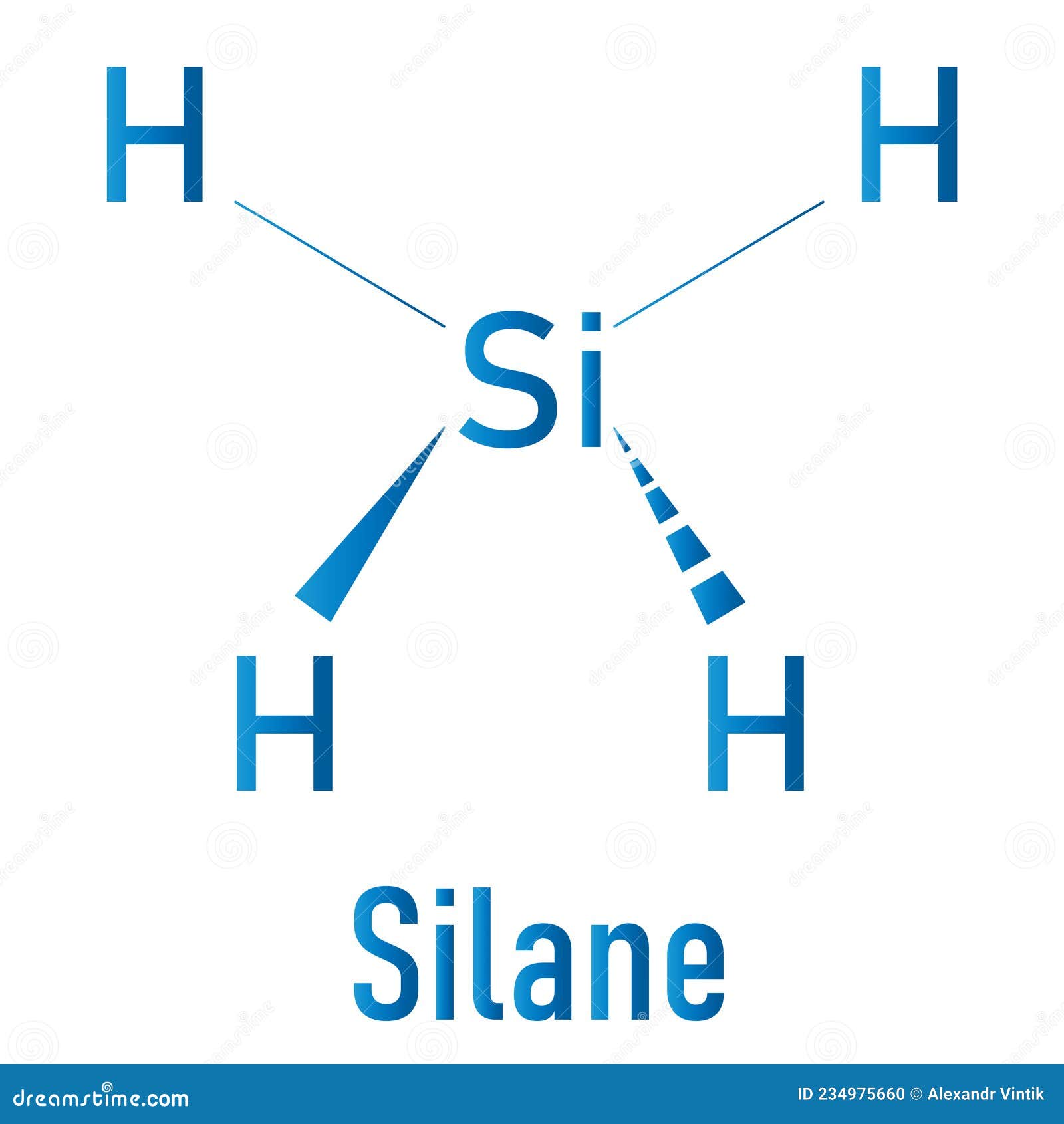
Silane SiH4 Molecule. Skeletal Formula Stock Vector Illustration of
Solved a Draw the Lewis structure for SiH4 in the window
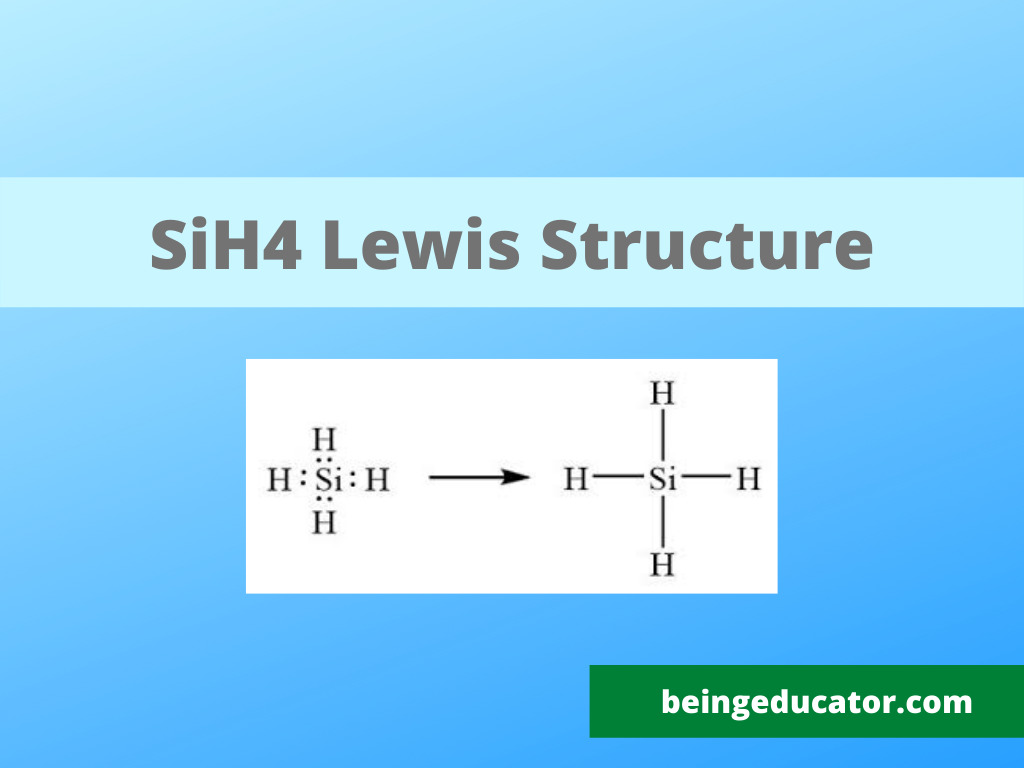
Lewis Structure, Hybridization, Polarity and Molecular Geometry of SiH4

diagrama de lewis del enlace siH4 Brainly.lat
[Solved] Draw the Lewis structure for SiH 4 in the window below and

SiH4 Lewis Structure (Silicon Tetrahydride) YouTube
Solved a Draw the Lewis structure for SiH4 in the window

Is SiH4 Polar or Nonpolar? Techiescientist
PPT Covalent Bonds PowerPoint Presentation, free download ID3048466

SiH4 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
Web 6 Steps To Draw The Lewis Structure Of Sih4 Step #1:
A Draw The Lewis Structure For Sih4 In The Window Below And Then Decide If The Molecule Is Polar Or Nonpolar.
This Problem Has Been Solved!
Okay, So You Asked To Draw The Loose Structure Of I H Four.
Related Post: