Draw The Lewis Structure For The Ammonia Nh3 Molecule
Draw The Lewis Structure For The Ammonia Nh3 Molecule - Connect the atoms with a lone pair: Understanding the molecular structure of ammonia (valence electrons are the electrons that are present in the outermost. Identifying the electron domain geometry and molecular geometry of nh3 (ammonia). Number of electron regions in ammonia. In, lewis’s structure of nh3, three bond pairs, and one lone pair are present. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drawing the lewis structure of nh3 (ammonia). Draw a lewis structure for ammonia (nh3). Each step of drawing the lewis structure of nh 3 is explained in detail in this tutorial. Number of electron regions in ammonia. Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. + this problem has been solved! Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web chemistry chemistry questions and answers writing the lewis structures for a molecule with resonance draw the lewis structure for the ammonia (nh,) molecule. Ammonia (nh3) lewis structure is made up of one nitrogen (n) atom and three hydrogens (h) atoms. For nh3, nitrogen. Web draw the lewis structure of ammonia (nh3). In, lewis’s structure of nh3, three bond pairs, and one lone pair are present. Draw a lewis structure for ammonia (nh3). Web steps involved in the nh3 lewis structure: In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen. This problem has been solved! Number of electron regions in ammonia. Web chemistry chemistry questions and answers draw a lewis structure for ammonia (nh3). Web the lewis structure of nh3, also known as ammonia, is a crucial concept in understanding the arrangement of atoms and electrons in a molecule. Web steps of drawing nh3 lewis structure step 1: Web chemistry chemistry questions and answers draw the lewis structure for the ammonia (nh3) molecule. Draw a lewis structure for ammonia (nh3). Web chemistry chemistry questions and answers writing the lewis structures for a molecule with resonance draw the lewis structure for the ammonia (nh,) molecule. After drawing the lewis structure of nh 3, you can decide shape of the. Drawing the lewis structure of nh3 (ammonia). In this section, we will explore the importance of identifying lone pairs in lewis structures , the number of lone pairs in nh3, and the effect of these lone pairs on the polarity of the compound. Draw a lewis structure for ammonia (nh3). + this problem has been solved! Web chemistry chemistry questions. After drawing the lewis structure of nh 3, you can decide shape of the nh 3 molecule. Drawing the lewis structure of nh3 (ammonia). To decide the geometry, shape and hybridization of a molecule, drawing the correct lewis structure is very important. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the. Web page contents show how to draw lewis structure of nh3 (ammonia)? Understanding the molecular structure of ammonia Be sure to include all resonance structures that satisfy the octet rule. Number of electron regions in ammonia. Web lewis structure of nh 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Be sure to include all resonance structures that satisfy the octet rule. Draw the lewis structure for the ammonia (nh3) molecule. Web the lewis structure of nh3, also known as ammonia, is a crucial concept in understanding the arrangement of atoms and electrons in a molecule. Drawing the lewis structure of nh3 (ammonia). + this problem has been solved! In order to draw the lewis structure of nh3, first of all you have to find the total number of valence electrons present in the nh3 molecule. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. Be sure to include. In this article, we will explore the nh3 lewis structure, also known as ammonia. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the nh3 molecule. Web chemistry chemistry questions and answers draw the lewis structure for the ammonia (nh3) molecule. Number of electron regions around nitrogen atom in. Web welcome to warren institute! Web in this video, you will learn how to draw the lewis structure for chemicals based on total valence electrons, the octet rule, duet rule, and also you will learn about lone pair and bonding pair. Count the total number of valence electrons to begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. Drawing the lewis structure for nh 3 ( ammmonia) Drawing the lewis structure of nh3 (ammonia). Web the lewis structure of nh3, also known as ammonia, is a crucial concept in understanding the arrangement of atoms and electrons in a molecule. Number of electron regions in ammonia. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web learn the steps to draw the lewis structure of nh3 (ammonia) in just 1 minute.📌you can draw any lewis structures by following the simple steps mentioned in. Identifying the electron domain geometry and molecular geometry of nh3 (ammonia). In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom.
NH3 (ammonia) Lewis dot structure YouTube
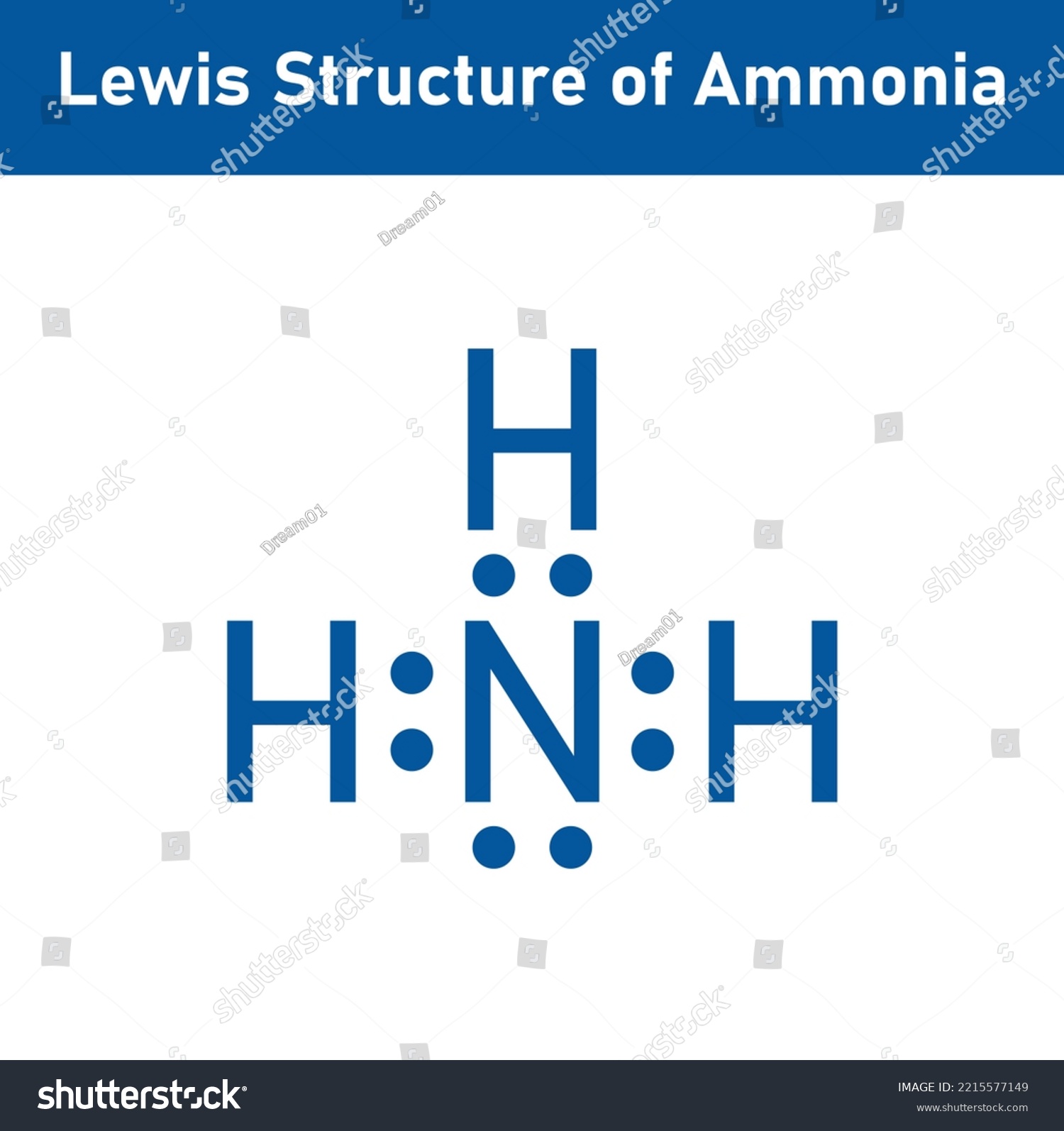
Lewis Structure Ammonia Nh3 Scientific Vector Stock Vector (Royalty

Lewis Dot Structure Of Nh3

Estructura de Lewis NH3, Amoniaco » Quimica Online

How to draw NH3 Lewis Structure? Science Education and Tutorials
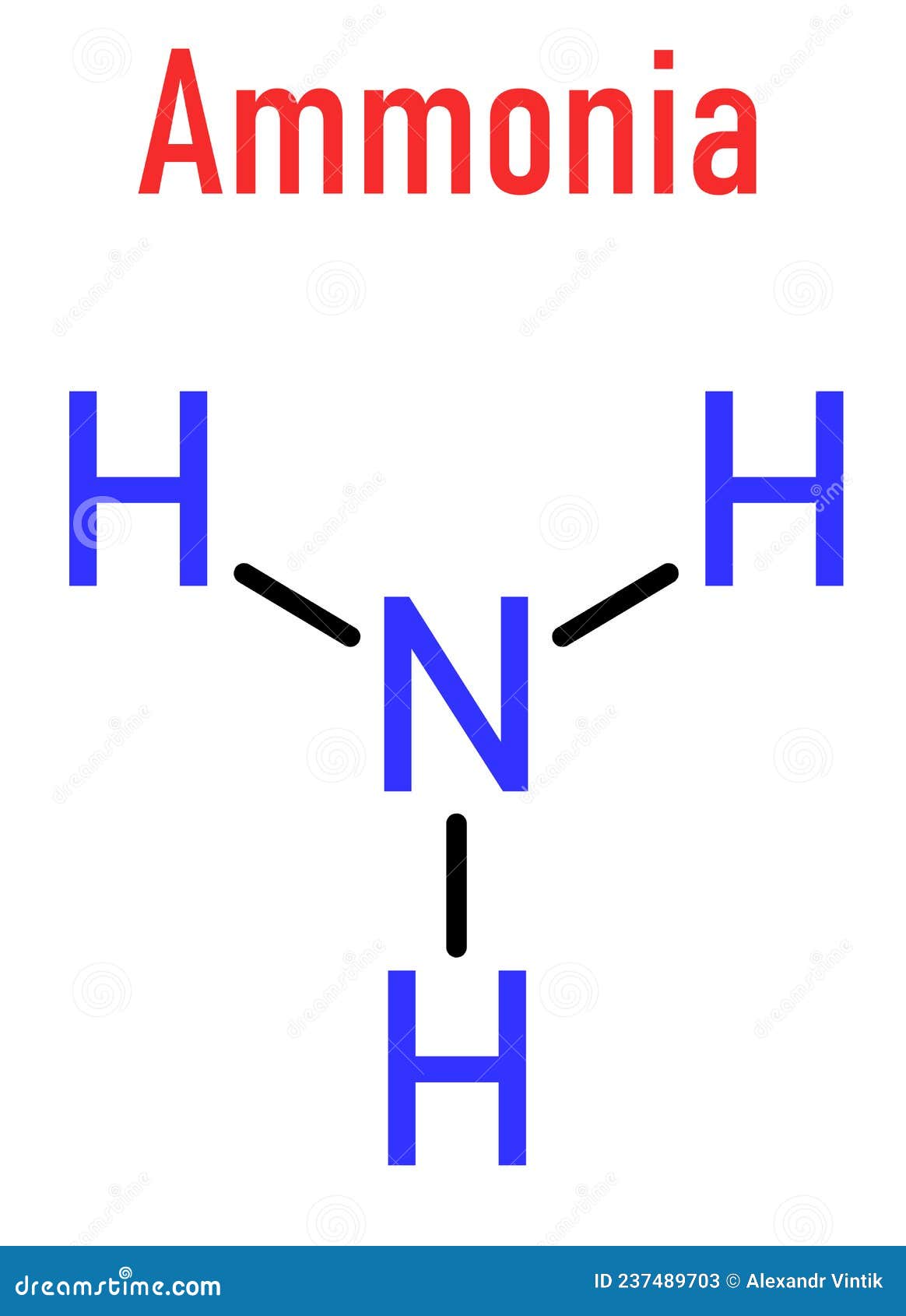
Ammonia NH3 Molecule. Skeletal Formula Stock Vector Illustration of

Nh3 ammonia molecule Royalty Free Vector Image

Draw the Lewis structure for ammonia, NH3. Include lone pairs. In the

Lewis Dot Diagram Of Nh3

Molécula Ammonia Nh3. Fórmula Esquelética. Ilustración del Vector
(Valence Electrons Are The Electrons That Are Present In The Outermost.
Draw The Lewis Structure For The Ammonia (Nh3) Molecule.
Web Ammonia Nh3 Lewis Dot Structure Shadowboy220 1.9K Subscribers Subscribe 16K Views 11 Years Ago Chemistry Lewis Dot Structures A Video Explanation Of How To Draw The Lewis Dot Structure.
For Nh3, Nitrogen (N) Is In Group 5A (Group 15), So It Has Five Valence Electrons.
Related Post: