Draw The Lewis Structure Of Ammonia Nh3
Draw The Lewis Structure Of Ammonia Nh3 - Web learn more understanding the nh3 lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the nh3 molecule. Connect the atoms with a lone pair: Web 6 steps to draw the lewis structure of nh3 step #1: Number of electron regions around nitrogen atom in ammonia Draw a single bond between each hydrogen atom and the nitrogen atom. Place two dots in between the spaces found in the h's and the n. Web draw the lewis structure of ammonia (nh3). Web ammonia nh3 lewis dot structure shadowboy220 1.9k subscribers subscribe 16k views 11 years ago chemistry lewis dot structures a video explanation of how to draw the lewis dot structure. Calculate the total number of valence electrons. How many bonding pairs are there around the n atom? Web to draw the nh3 lewis structure, follow these steps: Place the nitrogen atom in the center of the structure and the three hydrogen atoms around it. For resonance structures there must be a double or triple bond present, which is not the case. Now, write down h, n, and. Calculate the total number of valence electrons. Place the nitrogen atom in the center of the structure and the three hydrogen atoms around it. Find the total valence electrons in nh3 molecule in order to find the total valence electrons in nh3 molecule , first of all you should know the valence electrons present in nitrogen atom as. Web ammonia. It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone pairs. This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. Web to draw the nh3 lewis structure, follow these steps: This problem has been solved! Web ammonia nh3 lewis dot. How many bonding pairs are there around the n atom? It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone pairs. Now that we know the valence electrons for the molecule, we can predict its lewis structure. For the nh3 structure use the periodic table to find the. Web science chemistry chemistry questions and answers draw a lewis structure for ammonia (nh3). This chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia. In this article, we will explore the nh3 lewis structure, also known as ammonia. Web welcome to warren institute! The lewis structure of ammonia, n h 3, would be. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web 15k views 3 years ago. + this problem has been solved! Web ammonia (nh 3) lewis structure. Drawing the lewis structure for nh 3 ( ammmonia) It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone pairs. Web draw the lewis structure of ammonia (nh3). Web science chemistry chemistry questions and answers draw a lewis structure for ammonia (nh3). For resonance structures there must be a double or triple bond present, which is not. To decide the geometry, shape and hybridization of a molecule, drawing the correct lewis structure is very important. Web we draw lewis structures to predict: Web science chemistry chemistry questions and answers draw a lewis structure for ammonia (nh3). Determine the total number of valence electrons for all the atoms in nh3, which is 5 (for nitrogen) + (3 x. Total electron region is taken by the summation of sigma bonds and lone pairs around relevant atom. To decide the geometry, shape and hybridization of a molecule, drawing the correct lewis structure is very important. It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone pairs. Web welcome. Count total valence electrons in nh3 first of all, determine the valence electron that is available for drawing the lewis structure of nh 3 because the lewis diagram is all about the representation of valence electrons around atoms. It is important to accurately distribute the valence electrons, identify the central atom, calculate formal charges, and determine bond pairs and lone. Answer link have a look here. Moreover, the presence of a single lone pair of electrons on the nitrogen atom is responsible for the bent geometrical structure of the nh3 molecule. Web 15k views 3 years ago. Count total valence electrons in nh3 first of all, determine the valence electron that is available for drawing the lewis structure of nh 3 because the lewis diagram is all about the representation of valence electrons around atoms. There is really only one way to draw the lewis structure for ammonia (nh3). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Total electron region is taken by the summation of sigma bonds and lone pairs around relevant atom. Number of electron regions around nitrogen atom in ammonia Calculate the total number of valence electrons. Web we draw lewis structures to predict: For resonance structures there must be a double or triple bond present, which is not the case. The lewis structure of ammonia, n h 3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. Understanding the molecular structure of ammonia To decide the geometry, shape and hybridization of a molecule, drawing the correct lewis structure is very important. + this problem has been solved! How many bonding pairs are there around the n atom?
Ammonia NH3 Molecule. Skeletal Formula Stock Vector Illustration of

Nh3 ammonia molecule Royalty Free Vector Image

NH3 (ammonia) Lewis dot structure YouTube

How to draw NH3 Lewis Structure? Science Education and Tutorials
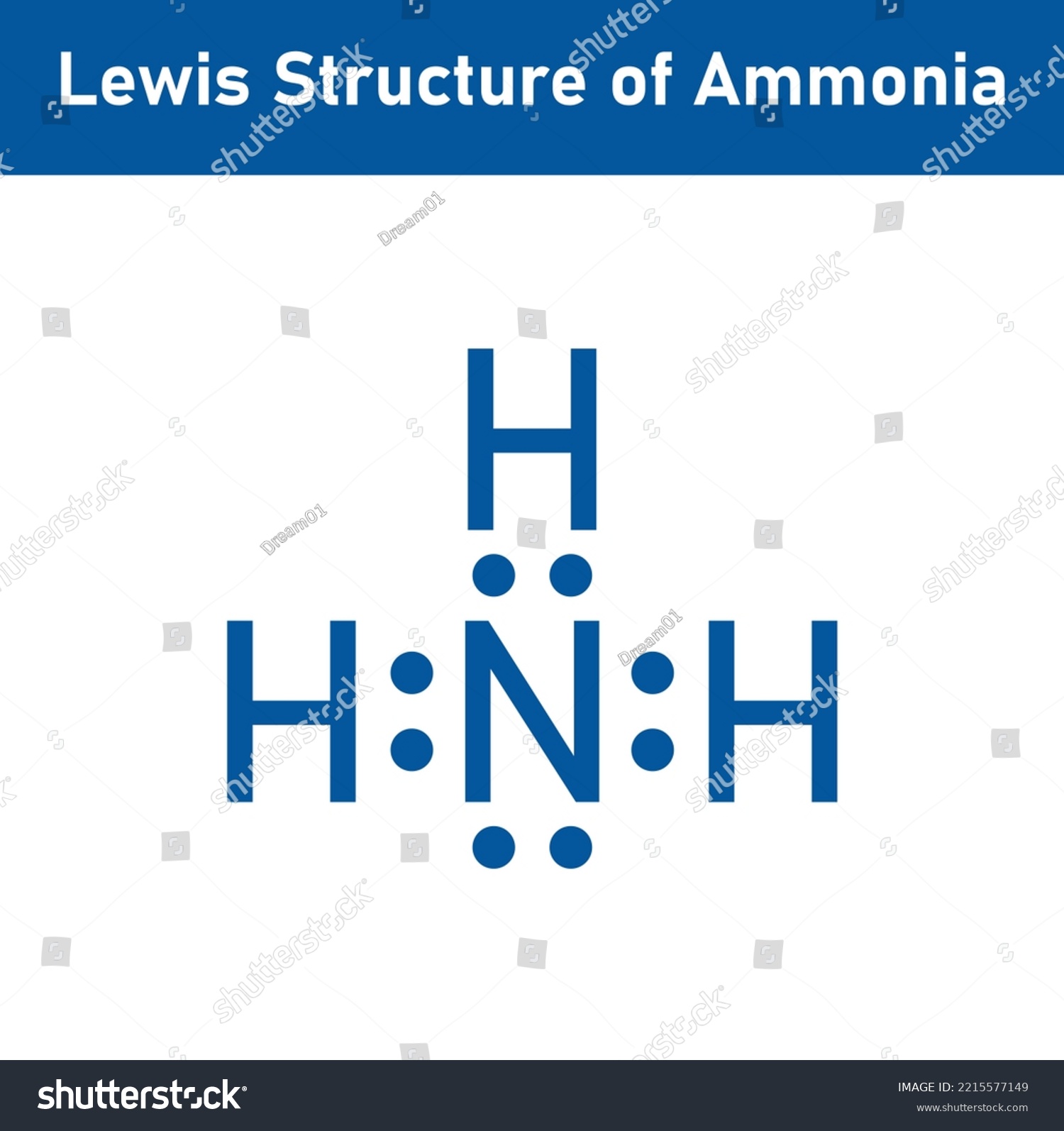
Lewis Structure Ammonia Nh3 Scientific Vector Stock Vector (Royalty

Estructura de Lewis NH3, Amoniaco » Quimica Online

Lewis Structure of NH3 (Ammonia) YouTube

NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube

Lewis Dot Diagram Of Nh3
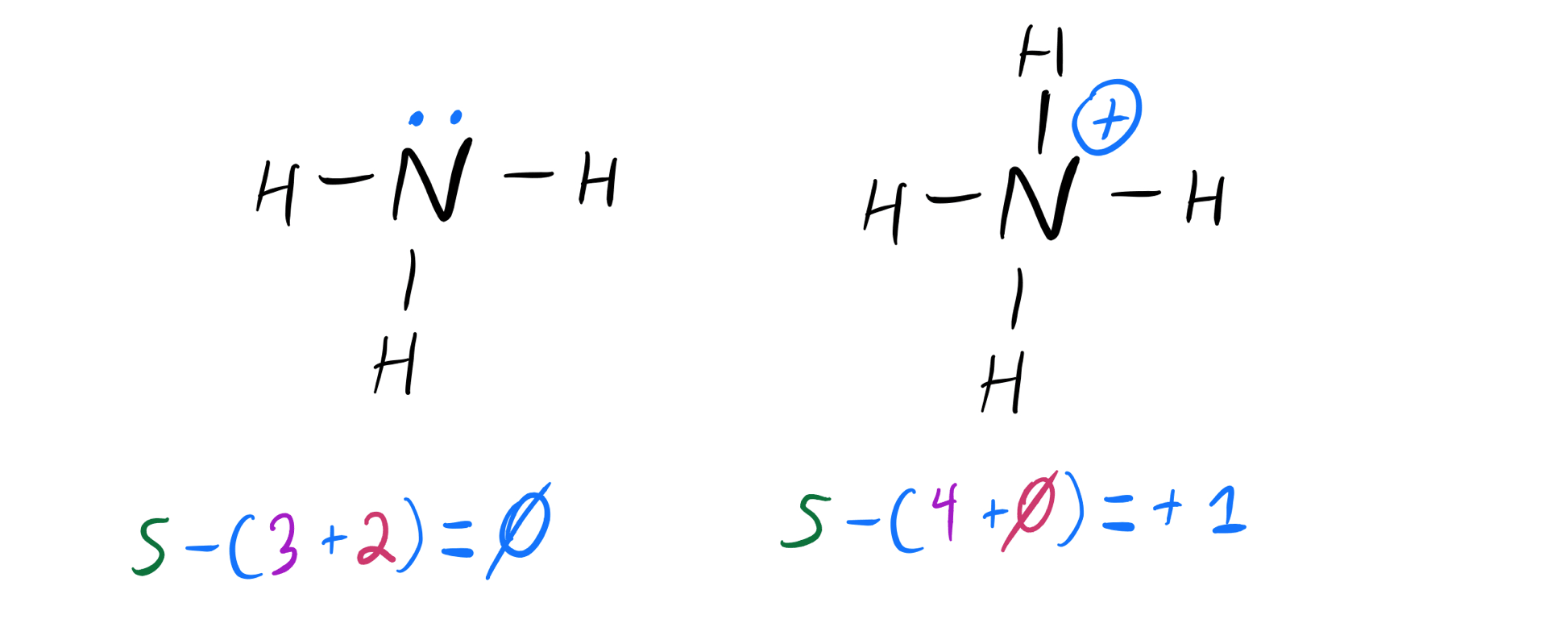
Draw the Lewis Structure for the Conjugate Acid of Ammonia En
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Web Science Chemistry Chemistry Questions And Answers Draw A Lewis Structure For Ammonia (Nh3).
This Problem Has Been Solved!
Determine The Total Number Of Valence Electrons For All The Atoms In Nh3, Which Is 5 (For Nitrogen) + (3 X 1) (For Hydrogen) = 8.
Related Post: