Draw The Lewis Structure Of Hf
Draw The Lewis Structure Of Hf - In hf, the fluorine atom is more electronegative than hydrogen and. Draw the lewis structure for sof_4. C is the central atom since it makes the most. Web to draw the lewis structure of hf, we follow a few simple steps: Also, helium is shown in group 8a, but it only has two valence electrons. Web how to draw the lewis structure of hf hydrogen fluoride?lewis structure: Key points to consider when drawing the hf electron dot structure. I also go over the shape and bond angle. Following the general procedure, now that we have found the valence. Web i quickly take you through how to draw the lewis structure of hf (hydrogen fluoride). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Valence electrons are the electrons in the outermost. Reference the “how to draw a. Draw lewis dot structures for ch4 ch 4, nh3 nh 3, hf hf, of2. Here, the given molecule is hf (hydrogen fluorine). #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around. Web here’s how you can easily draw the hf lewis structure step by step: Draw the lewis structure for hf draw the lewis structure for hf here’s the best way to solve it. Web how to draw hf lewis structure? I also go over the shape and bond angle. In the case of hf, we can represent the valence electrons. Web some structures don't obey the octet rule, but explain why. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web here’s how you can easily draw the hf lewis structure step by step: Construct a skeleton structure for the molecule. Valence electrons are the electrons in the outermost. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. For the hf structure use the periodic table to find the total number of. Select the center atom (h is always outside). Calculate the total number of valence electrons. With two bonding pairs and two. Draw a lewis structure for h_3ccoch_3. Draw the lewis structure for p 4. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms now, let’s take a closer look at each step mentioned above. Draw the lewis structure for the hccch_3 molecule. For the hf structure use the. Draw the lewis structure for sof_4. Draw the lewis structure for hf draw the lewis structure for hf here’s the best way to solve it. Web the lewis dot structure is a diagram that represents the valence electrons of an atom or molecule using dots. Web i quickly take you through how to draw the lewis structure of hf (hydrogen. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web how to draw the lewis structure of hf hydrogen fluoride?lewis structure: Web 67k views 10 years ago. Web the two atoms can share their unpaired electrons to make a covalent bond: Count the total. The central atom is fluorine, which is bordered on two terminals with hydrogen atoms ( in. Reference the “how to draw a. Web here’s how you can easily draw the hf lewis structure step by step: Web steps of drawing hf lewis structure step 1: Valence electrons are the electrons in the outermost. Draw the lewis structure for sof_4. Draw the lewis structure of chloroform (chcl3) and then determine the ideal bonding angle (s) of the central atom. Reference the “how to draw a. Here, the given molecule is hf (hydrogen fluorine). Also, helium is shown in group 8a, but it only has two valence electrons. Web 6 steps to draw the lewis structure of hf step #1: Web steps of drawing hf lewis structure step 1: In this video, we are. C is the central atom since it makes the most. Find the total valence electrons in hf molecule in order to find the total valence electrons in hf (hydrogen fluoride) molecule, first of all you should know the valence electrons present in a single hydrogen atom as well as fluorine atom. Count the total number of valence electrons in the molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. In hf, the fluorine atom is more electronegative than hydrogen and. Following the general procedure, now that we have found the valence. Web draw the lewis structure for i) co ii) xef4 iii) pcl3; Web i quickly take you through how to draw the lewis structure of hf (hydrogen fluoride). For the hf structure use the periodic table to find the total number of. Hydrogen has 1 valence electron and fluorine (in group 7 with f and cl) has 7 valence electrons. Web how to draw hf lewis structure? Web how to draw the lewis structure of hf hydrogen fluoride?lewis structure: Select the center atom (h is always outside).
So far, we’ve used 8 of the HF Lewis structure’s total 8 outermost

HF Lewis Structure, Molecular Geometry, Hybridization, and Polarity

Lewis dot structure of HF Hydrofluoric acid lewis dot structure
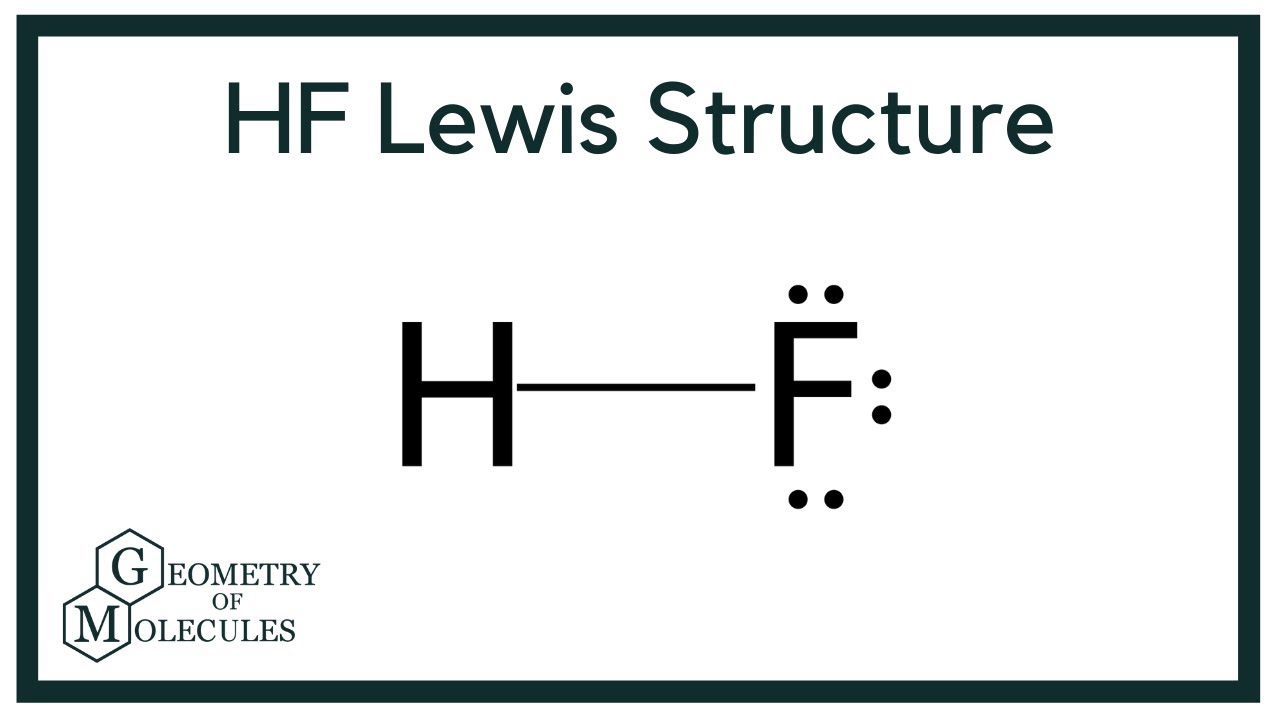
HF Lewis Structure (Hydrogen Fluoride) YouTube

Identify a bond between H and F in HF molecule Brainly.in
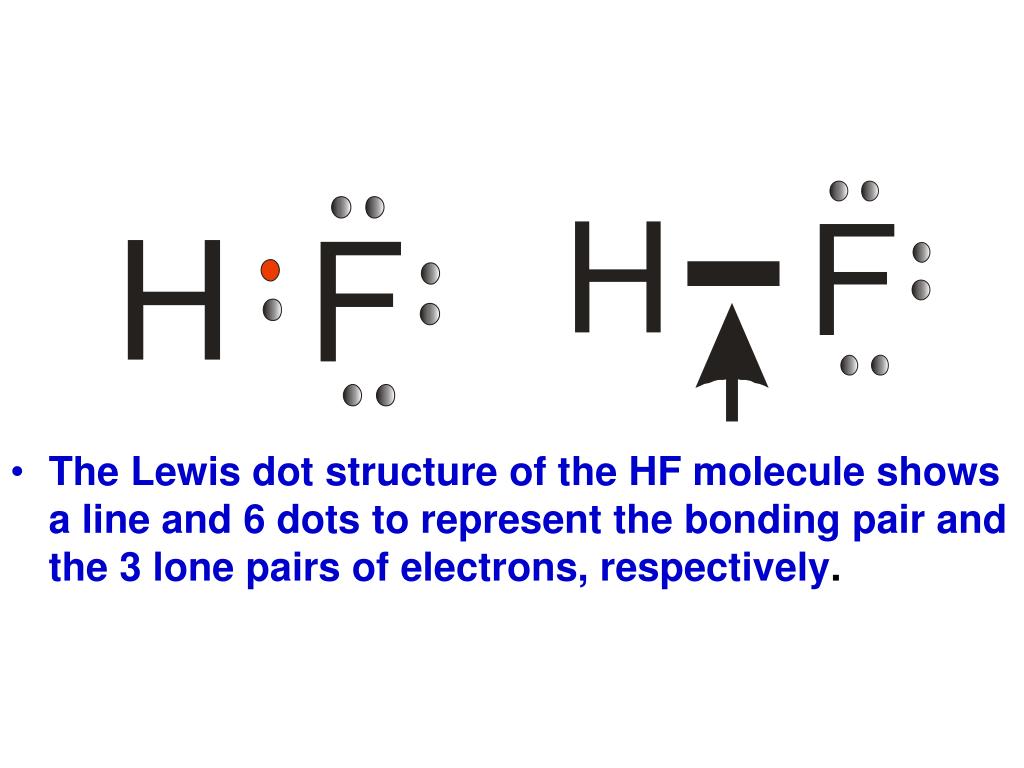
PPT Lewis Dot Structures of Covalent Compounds PowerPoint

How to draw HF Lewis Structure? Science Education and Tutorials

Draw the Lewis Structure of HF (hydrogen fluoride) YouTube

HF Molecular Geometry Science Education and Tutorials

Hydrogen fluoride Lewis structure Covalent bond Chemical bond, others
With The Lewis Structure For Hf Remember That Hydrogen Only Needs 2.
Web Here’s How You Can Easily Draw The Hf Lewis Structure Step By Step:
While Selecting The Atom, You Have To Put The Least.
Web We Can Illustrate The Formation Of A Water Molecule From Two Hydrogen Atoms And An Oxygen Atom Using Lewis Dot Symbols:
Related Post: