Draw The Orbital Diagram For Chlorine
Draw The Orbital Diagram For Chlorine - In the third column, draw the atomic diagram for your second element. The next six electrons will go in the 2p orbital. Determine the following (write the number answer only) number of unpaired electrons = number of valence electrons = number of core electrons = this problem has been solved! In an orbital diagram, an electron is represented by an arrow, while a box represents an atomic orbital. The p, d, and f orbitals have different sublevels, thus can hold more electrons. Web fluorine (atomic number 9) has only one 2p orbital containing an unpaired electron. The atomic number of chlorine represents the total number of electrons of chlorine. Web to visualize the electron configuration of chlorine, we can use an orbital diagram. Page contents show how to draw bohr model of chlorine (cl)? Each sublevel is labeled by its shell and sublevel. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Web draw the orbital diagram for chlorine. Web the chlorine orbital diagram is a graphical representation of the electron configuration. Web an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. There are three different rules used for constructing an atomic orbital diagram. To allow space for the next step, draw the orbitals further apart than you would for an atomic. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. In this diagram, each orbital is represented by a box, and the electrons are represented by arrows. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the. In an orbital diagram, the individual orbitals are shown as squares and orbitals within a sublevel are drawn next to each other horizontally. Web to visualize the electron configuration of chlorine, we can use an orbital diagram. To do that we need to find the number. Web the four different types of orbitals (s,p,d, and f) have different shapes, and. In this diagram, each orbital is represented by a box, and the electrons are represented by arrows. The atomic number of chlorine represents the total number of electrons of chlorine. Web to visualize the electron configuration of chlorine, we can use an orbital diagram. Web fluorine (atomic number 9) has only one 2p orbital containing an unpaired electron. Web the. Page contents show how to draw bohr model of chlorine (cl)? In an orbital diagram, the individual orbitals are shown as squares and orbitals within a sublevel are drawn next to each other horizontally. Bohr model describes the visual representation of orbiting electrons around the small nucleus. Since 1s can only hold two electrons the next 2 electrons for chlorine. The orbitals are 1s, 2s, 2p, 3s, and 3p. To allow space for the next step, draw the orbitals further apart than you would for an atomic orbital diagram. 3 20 orbital diagram sublevel labels 2s 4s 1s 30 reset. This diagram shows how the electrons in the chlorine atom are arranged in different orbitals. Web electron configuration diagram for. In an orbital diagram, an electron is represented by an arrow, while a box represents an atomic orbital. Web in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. View the full answer previous question next question An orbital diagram is the more visual way to represent the arrangement of all the electrons. The next six electrons will go in the 2p orbital. Web the orbital diagram or orbital notation simply represents the arrangement of electrons in different orbitals of an atom. Orbital is the region of space around the nucleus of an atom where electrons are found. 3 20 orbital diagram sublevel labels 2s 4s 1s 30 reset. Web steps find electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. The electron configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The electron configurations and orbital diagrams of these four elements are: Web in the first column, draw the atomic diagram for your. Bohr model describes the visual representation of orbiting electrons around the small nucleus. (b) cl draw the orbital diagram for each of the following: In an orbital diagram, an electron is represented by an arrow, while a box represents an atomic orbital. Web the orbital diagram or orbital notation simply represents the arrangement of electrons in different orbitals of an atom. Web the chlorine orbital diagram is a graphical representation of the electron configuration of the chlorine atom. Web in writing the electron configuration for chlorine the first two electrons will go in the 1s orbital. Web what is the orbital diagram for chlorine (cl)? The p, d, and f orbitals have different sublevels, thus can hold more electrons. 3 20 orbital diagram sublevel labels 2s 4s 1s 30 reset. So to draw the ground state atomic orbital diagram, we need to fill these 17 electrons in the orbitals of cl according to hund's rule and, auf bau principle. Note that there may be extra answer choices and/or extra answer boxes that remain unused in the correct answer. Now in the next step,. Page contents show how to draw bohr model of chlorine (cl)? This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above. Web an orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. Web an orbital box diagram can be written as well.
Chlorine Cl (Element 17) of Periodic Table NewtonDesk
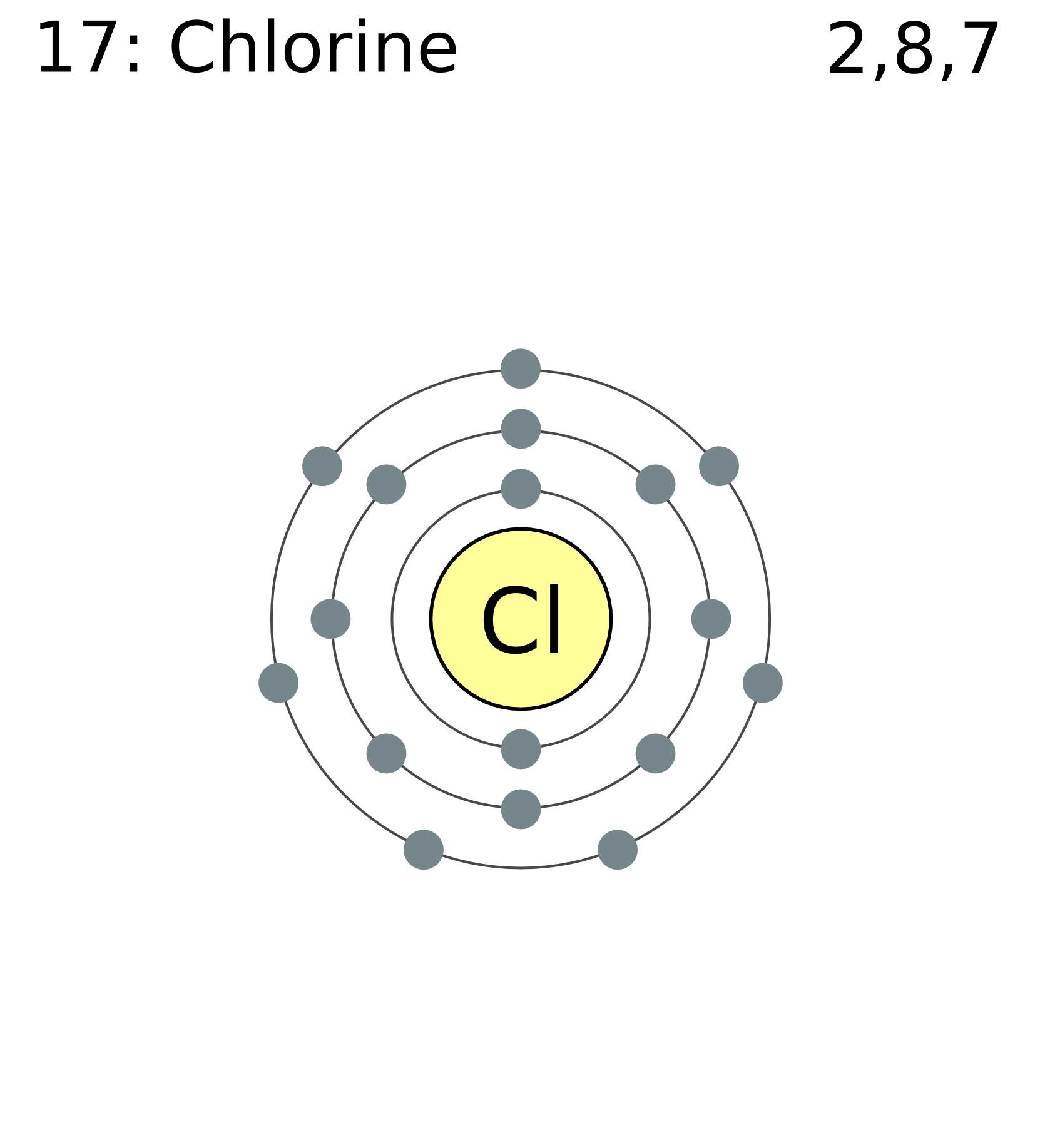
FileElectron shell 017 chlorine.png Wikimedia Commons
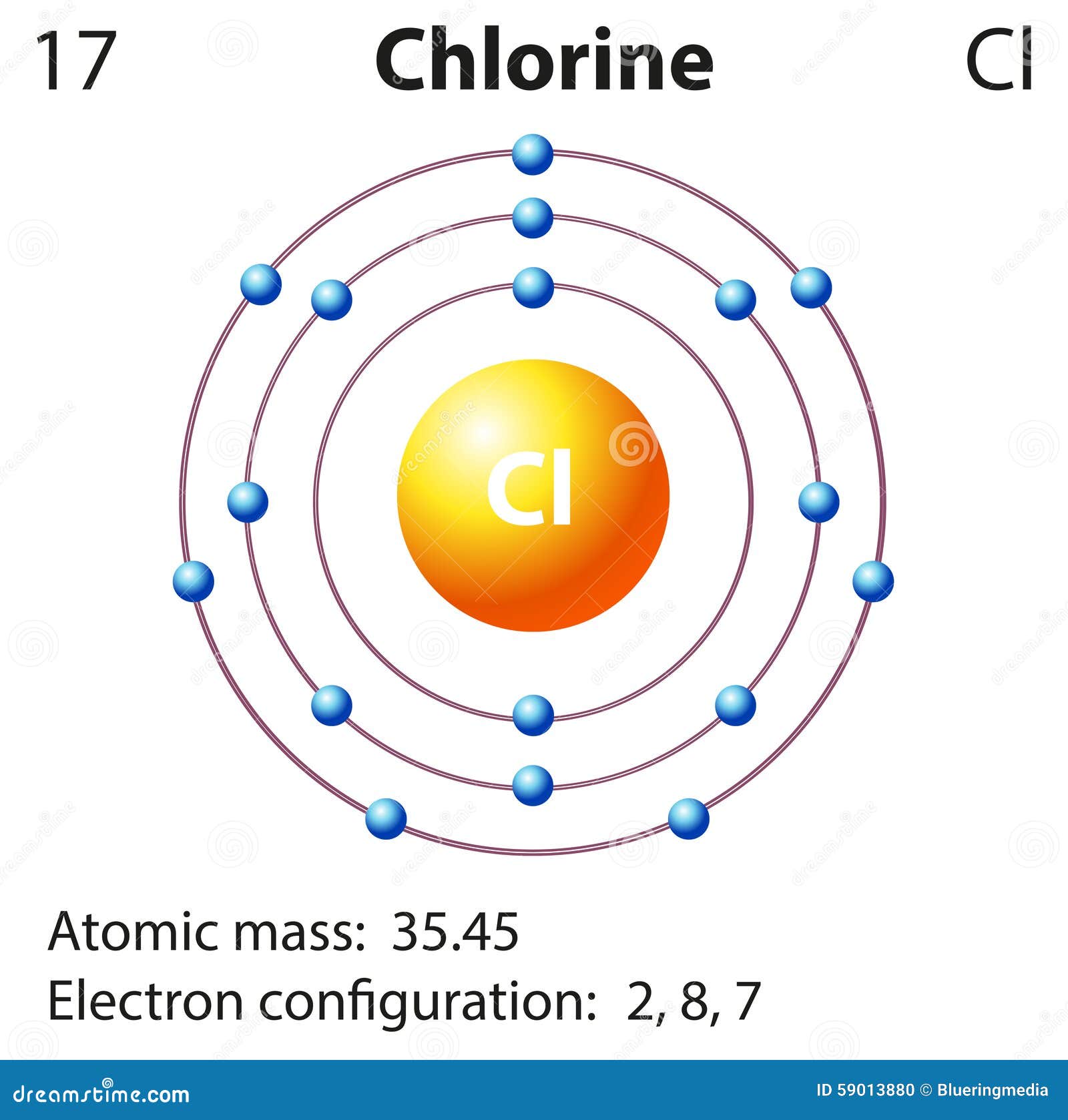
Diagram Representation of the Element Chlorine Stock Illustration

Electron Configuration For Chlorine
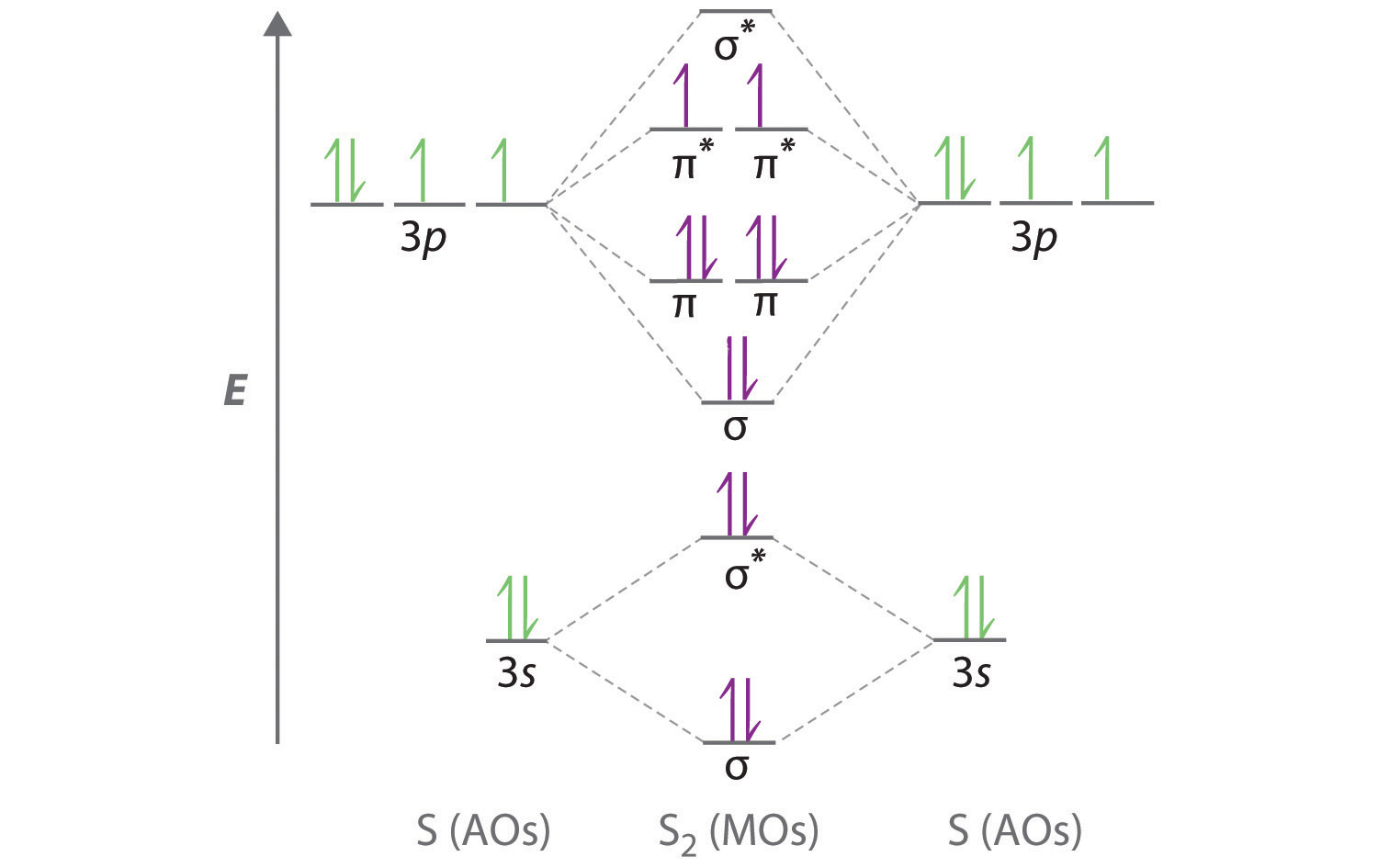
Molecular Orbital Diagram For Cl2
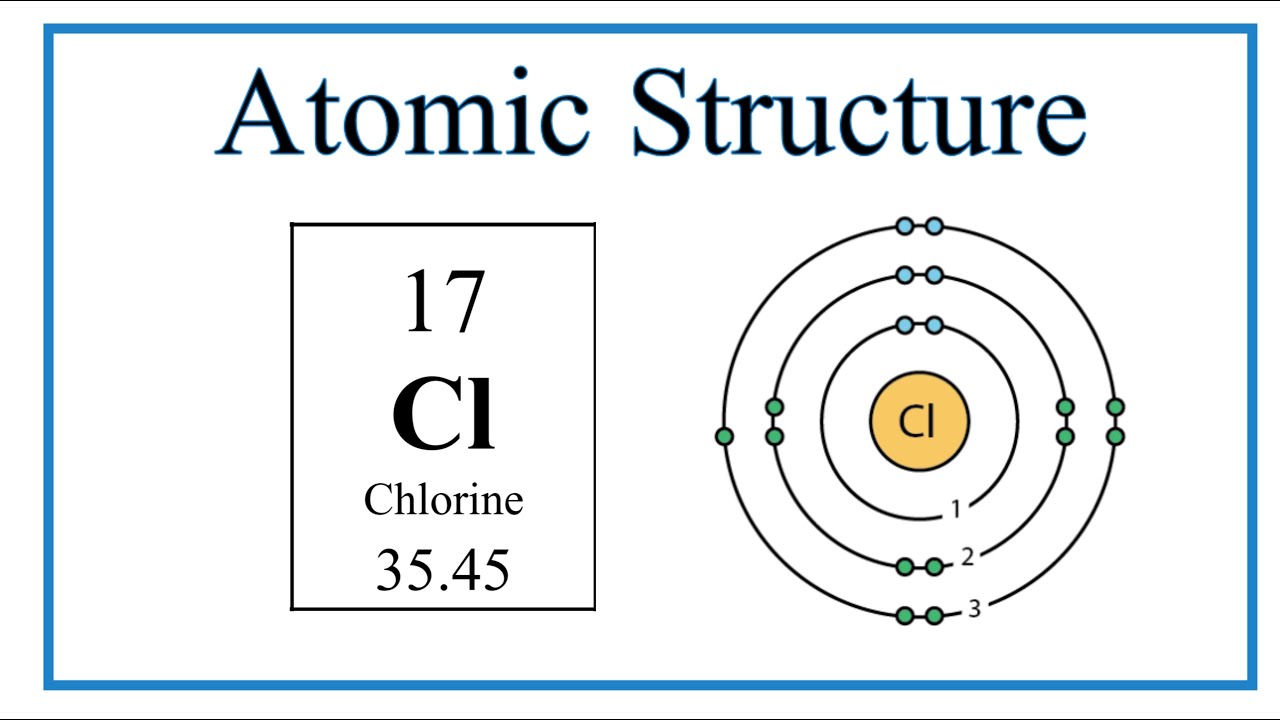
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube

Chlorine electron configuration Stock Image C029/5025 Science
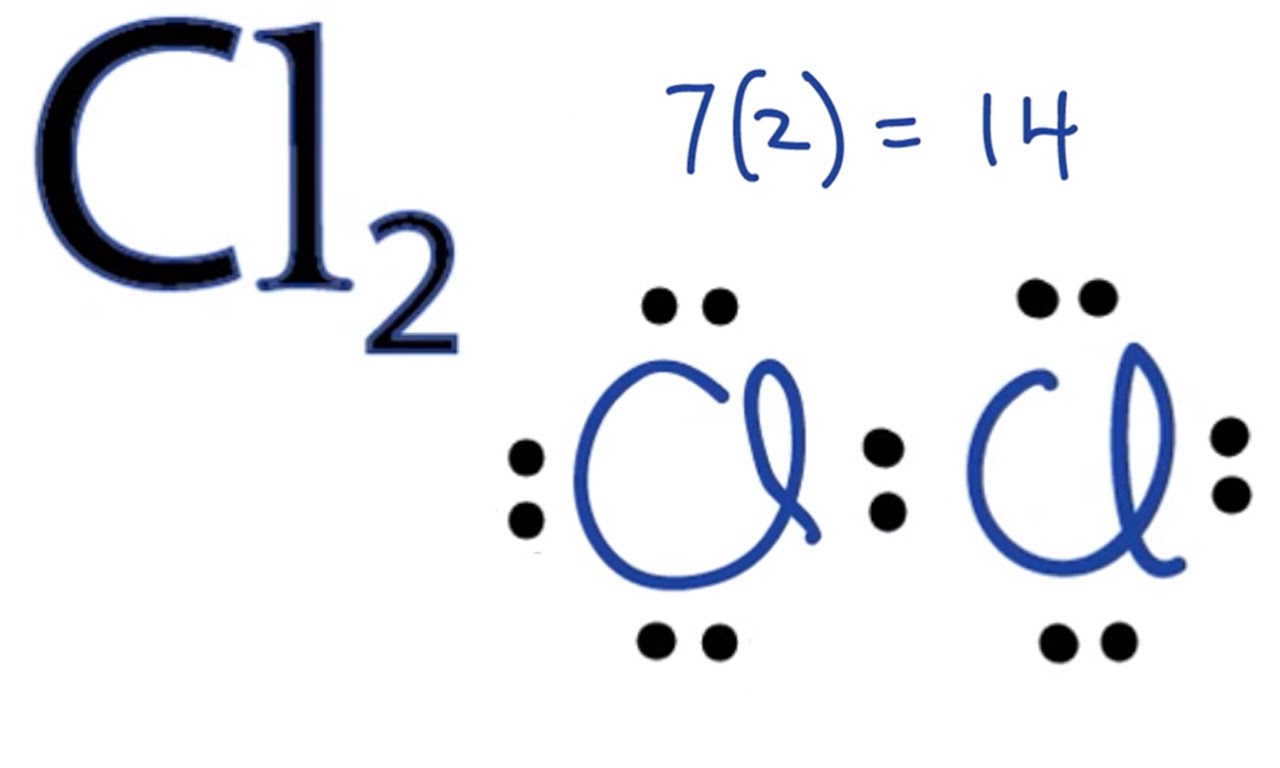
Chlorine Electron Configuration (Cl) with Orbital Diagram
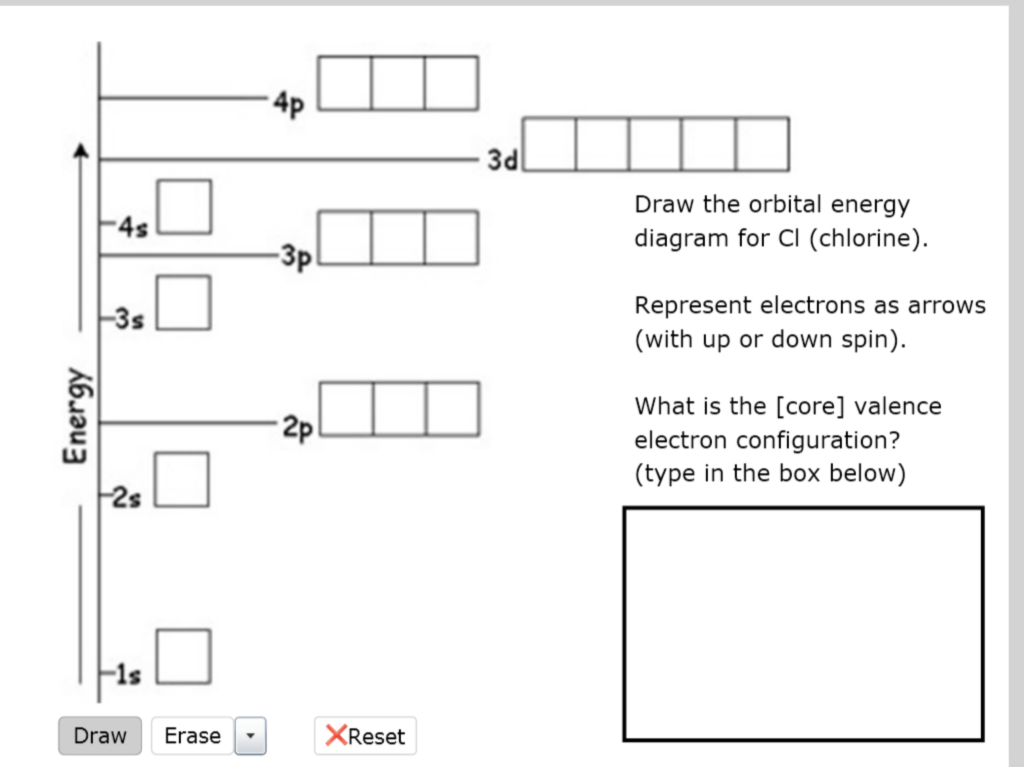
Solved 4p 3d Draw the orbital energy diagram for Cl
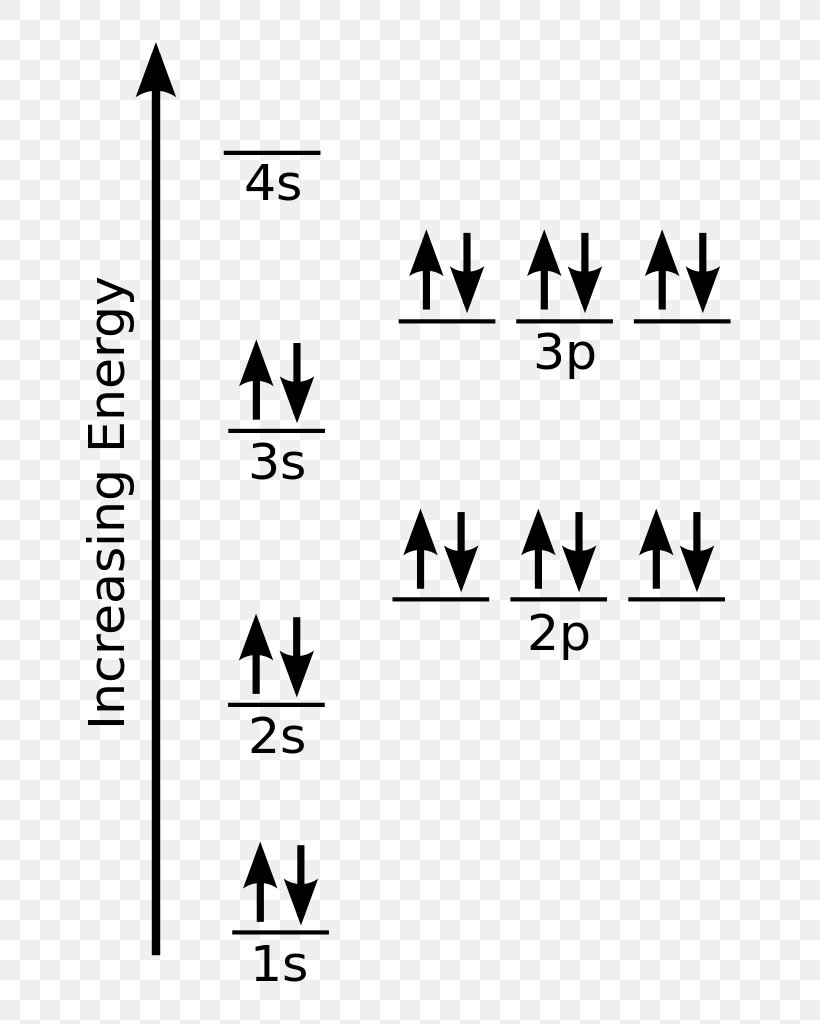
Electron Configuration Atomic Orbital Chlorine Chemistry, PNG
As Stated, The Electron Configuration Of Each Element Is Unique To Its Position On The Periodic Table.
We Start With A Single Hydrogen Atom (Atomic Number 1), Which Consists Of One Proton And One Electron.
The Orbital Diagram For Chlorine Would Show The Filling Of The 1S, 2S, 2P, 3S, And 3P Orbitals According To Its Electron Configuration.
The Orbital Diagram For Chlorine Is Drawn With 5 Orbitals.
Related Post: