Draw The Organic Product For The Following Acid-Catalyzed Hydrolysis Reaction
Draw The Organic Product For The Following Acid-Catalyzed Hydrolysis Reaction - This problem has been solved! In this case, the ester is methyl benzoate, which has the formula c6h5cooch3. This reaction is typically used to break apart complex organic molecules, such as carbohydrates, proteins, and fats, into their constituent parts, which can then be further. Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. Phenylacetaldehyde + hydrazine • you do not have to consider stereochemistry. Use the wedge and hash bond tools to indicate stereochemistry where it exists. Web question transcribed image text: Web first, we have to identify the functional group of the reactant. Web this page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as hydrochloric acid or sulfuric acid) acting as the catalyst. Web this problem has been solved! The starting material is an acetal. • apply formal charges to any nitro groups. Draw the major organic product (s) of the following reactions including stereochemistry when it is appropriate. Draw the organic intermediate after each elementary step. Step by step solved in 2 steps with 2 images see solution check out a sample q&a here knowledge booster learn more. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The reactant is an ester, which has the general formula rcoor'. H2so4, h20 expert solution trending now this is a popular solution! Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the. Web organic chemistry i 6: Nomenclature and reactions of carboxylic acid derivatives nomenclature and properties of acyl (acid) halides and acid anhydrides nomenclature and properties of esters nomenclature and properties of amides reactivity of carboxylic acid derivatives nucleophilic acyl substitution You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Like esterification, the reaction. Draw a structural formula for the organic product of the following acid catalyzed reaction. Web draw a structural formula for the organic product of the following acid catalyzed reaction. Nomenclature and reactions of carboxylic acid derivatives nomenclature and properties of acyl (acid) halides and acid anhydrides nomenclature and properties of esters nomenclature and properties of amides reactivity of carboxylic acid. Web organic chemistry i 6: Draw the organic intermediate after each elementary step. Web question transcribed image text: Do not draw inorganic byproducts. Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. It uses ethyl ethanoate as a typical ester. Step by step solved in 2 steps with 2 images see solution check out a sample q&a here knowledge booster learn more about Nomenclature and reactions of carboxylic acid derivatives nomenclature and properties of acyl (acid) halides and acid anhydrides nomenclature and properties of esters nomenclature and properties of amides reactivity of. Here’s the best way to. Web in organic chemistry, acid hydrolysis can be described as a hydrolysis process in which a protic acid is utilized to catalyze the cleavage of a chemical bond through a nucleophilic substitution reaction, with the addition of water (h₂o). Nomenclature and reactions of carboxylic acid derivatives nomenclature and properties of acyl (acid) halides and acid. Web draw a structural formula for the organic product of the following acid catalyzed reaction. This problem has been solved! Web first, we have to identify the functional group of the reactant. Think about which bonds will be broken in. Like esterification, the reaction is reversible and does not go to completion. Web organic chemistry i 6: The reactant is an ester, which has the general formula rcoor'. Web question transcribed image text: The starting material is an acetal. Like esterification, the reaction is reversible and does not go to completion. Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. This reaction is typically used to break apart complex organic molecules, such as carbohydrates, proteins, and fats, into their constituent parts, which can. Web organic chemistry i 6: H2so4, h20 i i h 5th attempt x incorrect @ see page 897 incorrect. Use the wedge and hash bond tools to indicate stereochemistry where it exists. Web the mechanism for the acid catalyzed hydrolysis reaction begins with protonation of the carbonyl oxygen to increase the reactivity of the ester. H2so4, h20 expert solution trending now this is a popular solution! This reaction is typically used to break apart complex organic molecules, such as carbohydrates, proteins, and fats, into their constituent parts, which can then be further. Organic chemistry > unit 11 lesson 4: Acidic hydrolysis is simply the reverse of esterification. For example, in the conversion of cellulose to glucose. Nomenclature and reactions of carboxylic acid derivatives nomenclature and properties of acyl (acid) halides and acid anhydrides nomenclature and properties of esters nomenclature and properties of amides reactivity of carboxylic acid derivatives nucleophilic acyl substitution Think about which bonds will be broken in. Draw the organic intermediate after each elementary step. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web question transcribed image text: The ph for reactions which form imine compounds must be carefully controlled. Like esterification, the reaction is reversible and does not go to completion.
Solved Draw The Organic Product For The Following Acidca...
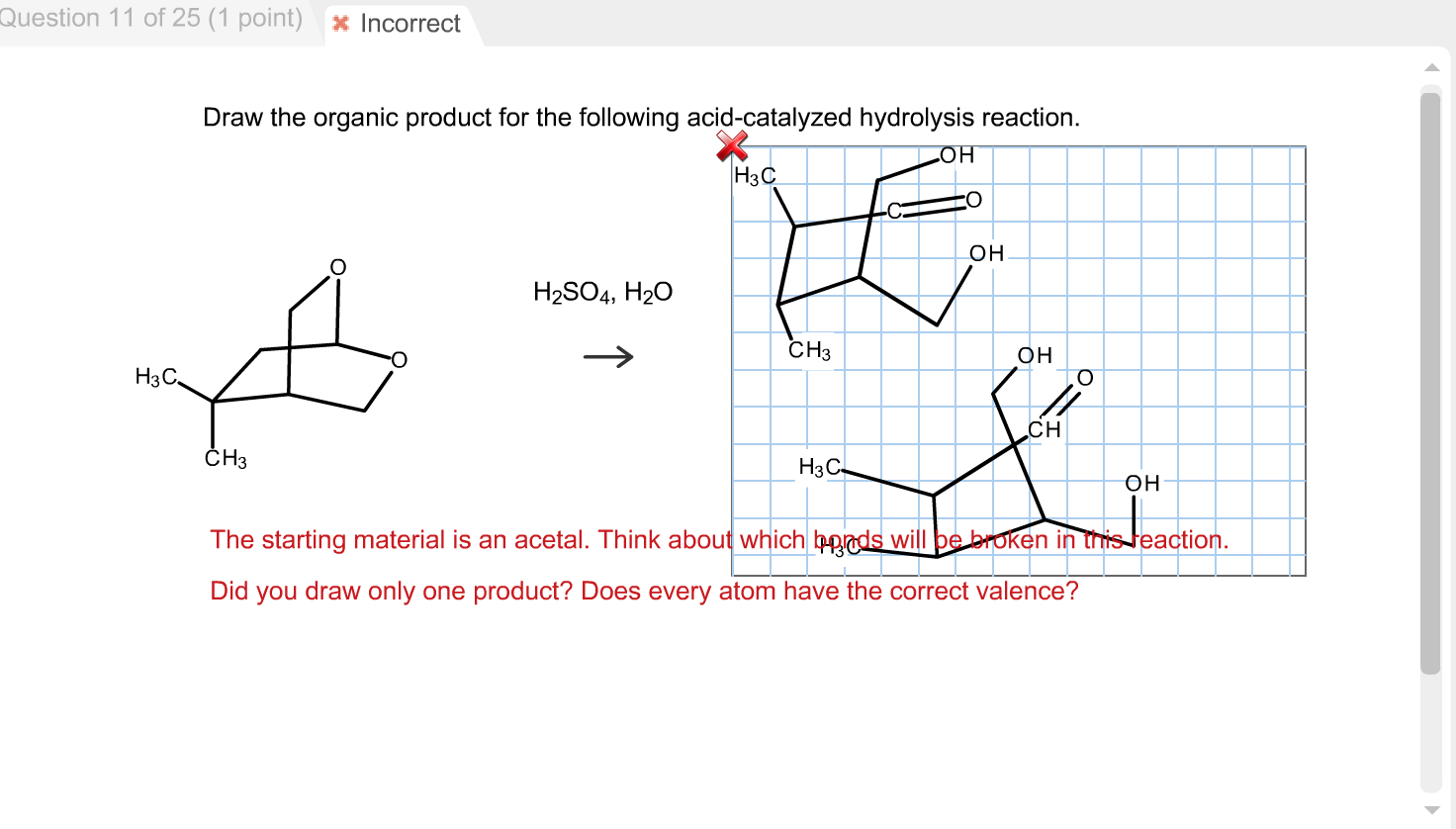
Solved Draw The Organic Product For The Following Acidca...
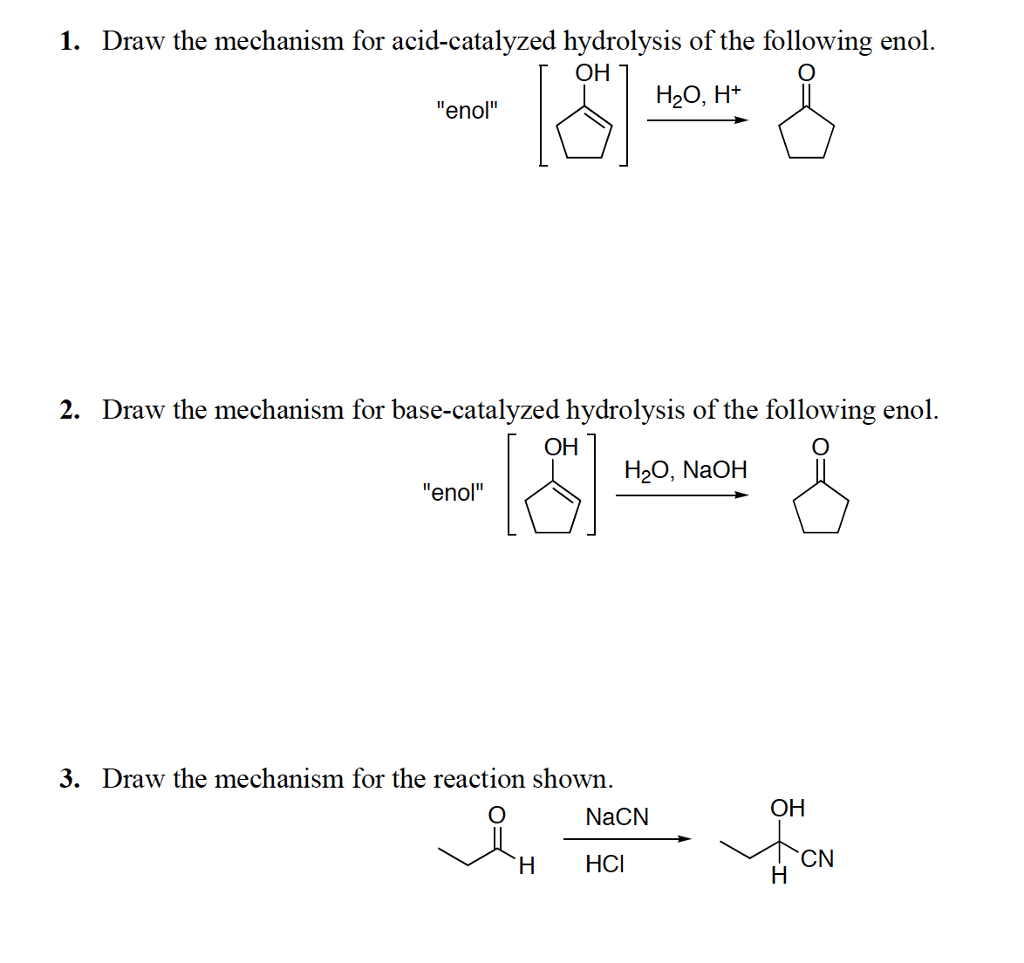
Solved 1. Draw the mechanism for acidcatalyzed hydrolysis

OneClass draw the organic product for the following acidcatalyzed
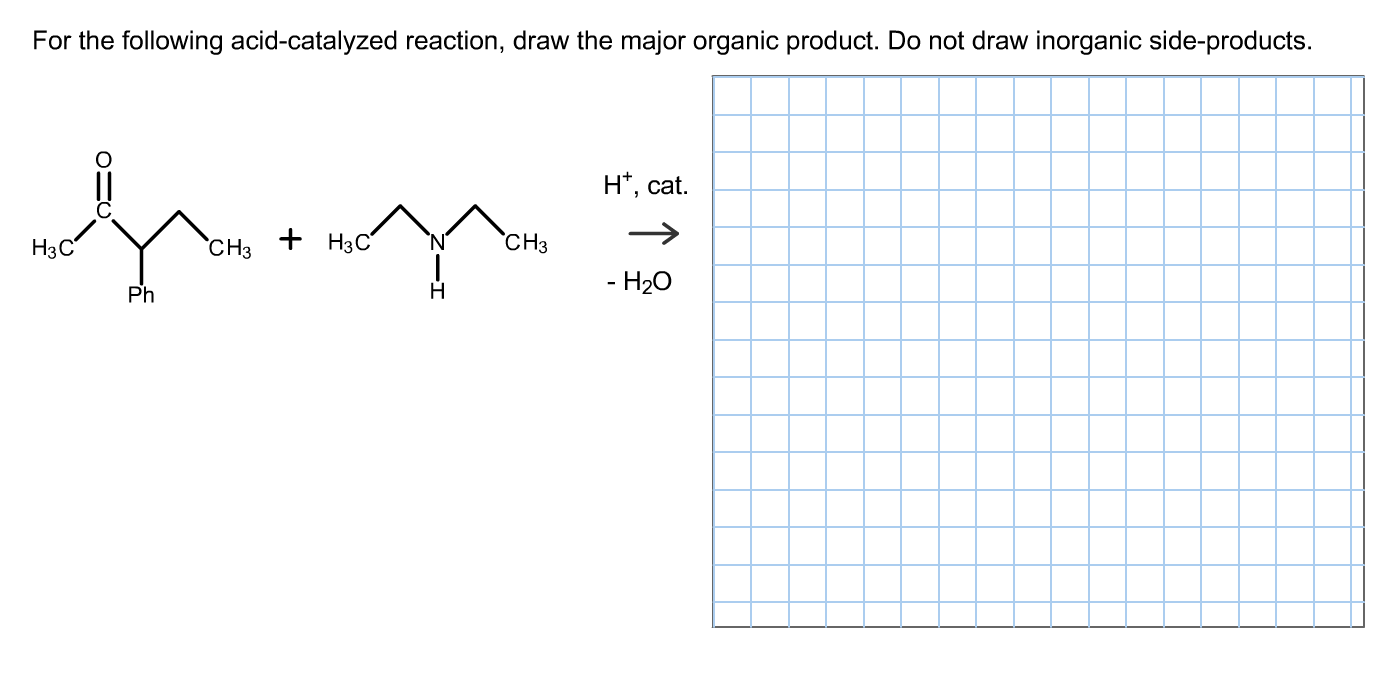
Solved For The Following Acidcatalyzed Reaction, Draw Th...
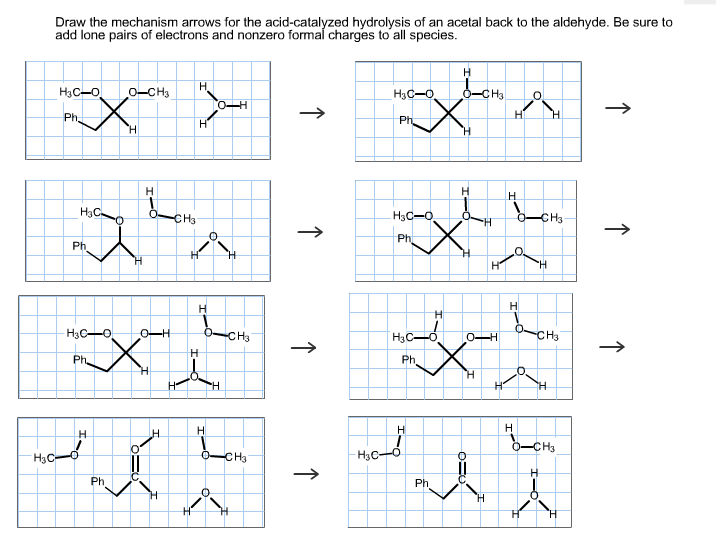
Solved Draw The Mechanism Arrows For The Acidcatalyzed H...
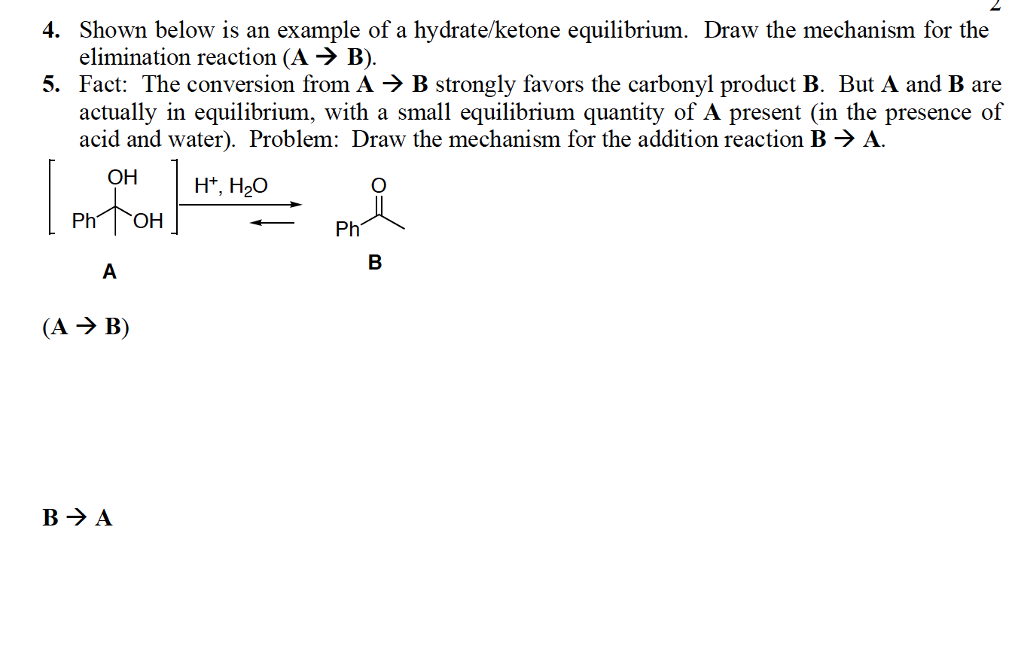
Solved 1. Draw the mechanism for acidcatalyzed hydrolysis

Draw the organic product for the following acidcatalyzed hydrolysis
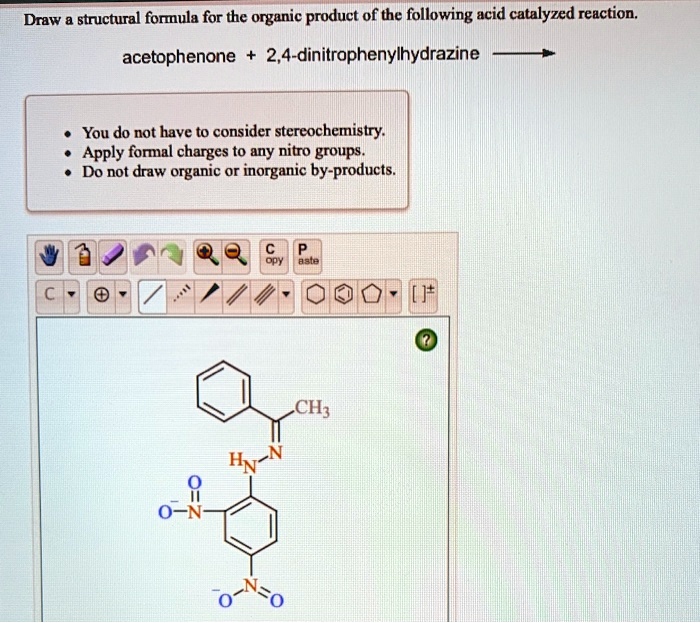
SOLVED Draw structural formula for the organic product of the
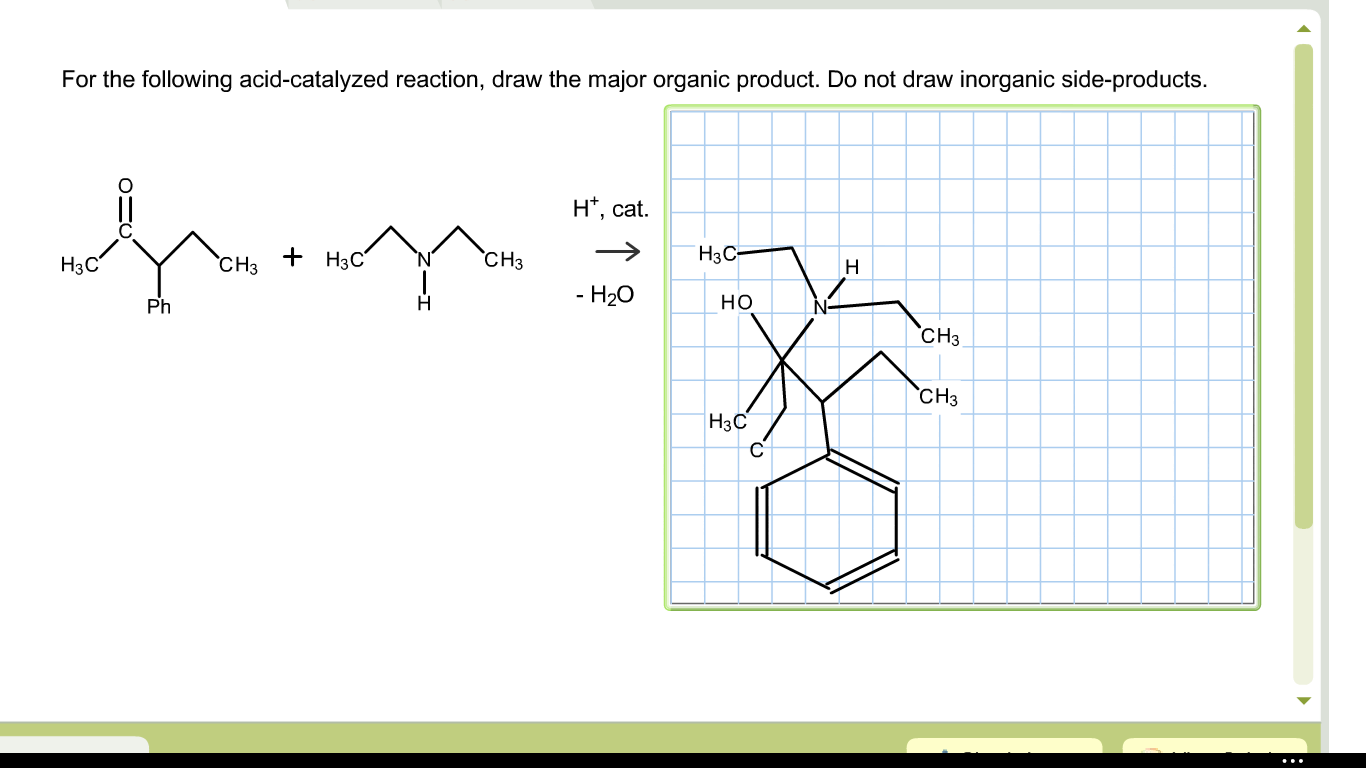
Solved For The Following Acidcatalyzed Reaction, Draw Th...
The Nucleophilic Water Reacts With The Electrophilic Carbonyl Carbon Atom To Form The Tetrahedral Intermediate.
Web First, We Have To Identify The Functional Group Of The Reactant.
• Apply Formal Charges To Any Nitro Groups.
The Hydrolysis Of The Hydroxyderivative Of Thp Would Happen As Follows.
Related Post: