Draw The Structure Of Aniline
Draw The Structure Of Aniline - See functional group, molecular weight, properties, basicity, reaction, and uses of aniline. The compound is a derivative of aniline. Web 1 structure of anilines 1.1 physical properties of anilines 2 faqs on anilines structure of anilines aniline is a slightly pyramidalized molecule. Here i show how to create them all by pushing electrons around.check me out: You need to understand about the bonding in benzene in order to make sense of this next bit. Web what is aniline? The nitrogen lone pair in the compound is in an spx hybrid orbital with a high p character. In the second resonance structure, the lone pair of electrons on the nitrogen atom is not used. Draw the structure of aniline. Draw the structure for each compound. In the first resonance structure, the amino group’s lone pair of electrons is used to form a bond with the carbon atom in the aromatic ring. Consisting of a phenyl group ( −c 6 h 5 ) attached to an amino group ( −nh 2 ), aniline is the simplest aromatic amine. See functional group, molecular weight, properties, basicity, reaction,. C 6 h 7 n. Web we have now applied this technique to an evaluation of the structure of the aniline molecule. It has hybridization of the nitrogen which is between sp3 and sp2. Draw the structure for each compound. It is an organic compound that consists of a phenyl group attached to an amine group. Consisting of a phenyl group ( −c 6 h 5 ) attached to an amino group ( −nh 2 ), aniline is the simplest aromatic amine. This problem has been solved! Web we have now applied this technique to an evaluation of the structure of the aniline molecule. C 6 h 7 n. It is a primary amine having an. In the second resonance structure, the lone pair of electrons on the nitrogen atom is not used. Draw the structure for each compound. Its a simple way to draw all resonating structure of molecule. Web aniline, also known as aminobenzene or phenylamine, has 6 carbon (c) atoms, 7 hydrogen (h) atoms, and 1 nitrogen (n) atom in its chemical formula. In the second resonance structure, the lone pair of electrons on the nitrogen atom is not used. Find the formula and structure of aniline. The lone pair overlaps with the delocalised ring electron system Draw the structure for each compound. The nitrogen lone pair in the compound is in an spx hybrid orbital with a high p character. Web we have now applied this technique to an evaluation of the structure of the aniline molecule. C 6 h 7 n. This problem has been solved! It has hybridization of the nitrogen which is between sp3 and sp2. Web what is aniline? There is an interaction between the delocalised electrons in the benzene ring and the lone pair on the nitrogen atom. Web structure of aniline the molecule of aniline has a pyramidal shape with a nitrogen hybridization that resides between sp3 and sp2. Here presence of bromine atoms in tribroboaniline, reduces the basic properties of the amino group, and salts even. Here presence of bromine atoms in tribroboaniline, reduces the basic properties of the amino group, and salts even with strong acids are. Web chemistry chemistry questions and answers draw the structure of aniline. Web q1 (i) draw the resonating structure of ozone molecule (ii) draw the resonating structure of nitrate ion view solution q2 which one of the following is. The nitrogen lone pair in the compound is in an spx hybrid orbital with a high p character. Expert answer 100% (2 ratings) step 1 In the second resonance structure, the lone pair of electrons on the nitrogen atom is not used. The lone pair overlaps with the delocalised ring electron system In the first resonance structure, the amino group’s. It has hybridization of the nitrogen which is between sp3 and sp2. See functional group, molecular weight, properties, basicity, reaction, and uses of aniline. You need to understand about the bonding in benzene in order to make sense of this next bit. Expert answer 100% (2 ratings) step 1 This problem has been solved! As a result, the nitrogen lone pair is in an spx hybrid orbital with a high p character. Web structure of aniline the molecule of aniline has a pyramidal shape with a nitrogen hybridization that resides between sp3 and sp2. Web this vedio will be helpful for understanding the resonating structures of aniline. Web chemistry chemistry questions and answers draw the structure of aniline. There is an interaction between the delocalised electrons in the benzene ring and the lone pair on the nitrogen atom. Web in this short video you will know how to draw resonating structure of aniline in chemdraw ultra and how to copy this structure to ms word and convert to pdf format. The nitrogen lone pair in the compound is in an spx hybrid orbital with a high p character. Expert answer 100% (2 ratings) step 1 Consisting of a phenyl group ( −c 6 h 5 ) attached to an amino group ( −nh 2 ), aniline is the simplest aromatic amine. It is an organic compound that consists of a phenyl group attached to an amine group. Draw the structure for each compound. The compound is a derivative of aniline. In the first resonance structure, the amino group’s lone pair of electrons is used to form a bond with the carbon atom in the aromatic ring. In the second resonance structure, the lone pair of electrons on the nitrogen atom is not used. It has hybridization of the nitrogen which is between sp3 and sp2. You need to understand about the bonding in benzene in order to make sense of this next bit.
Aniline organic solvent molecular structure Vector Image
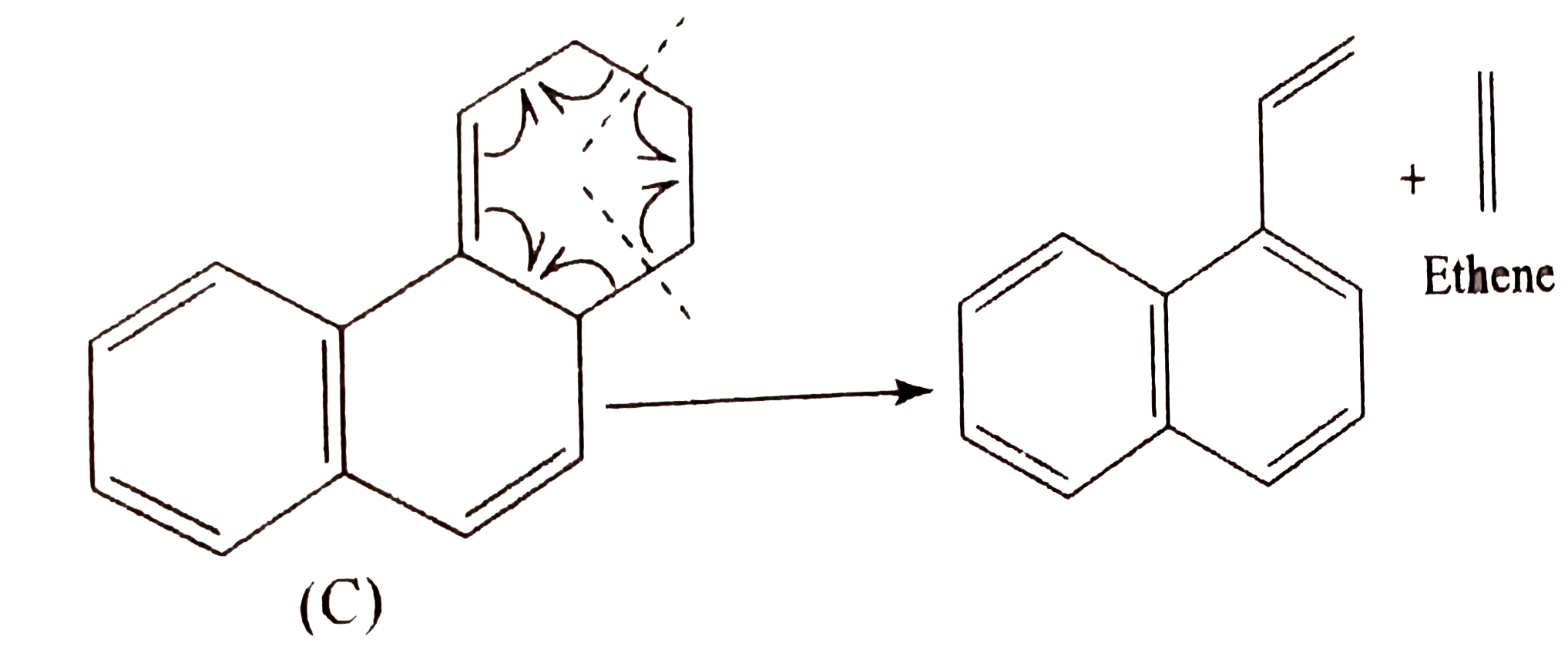
Draw the structure of aniline
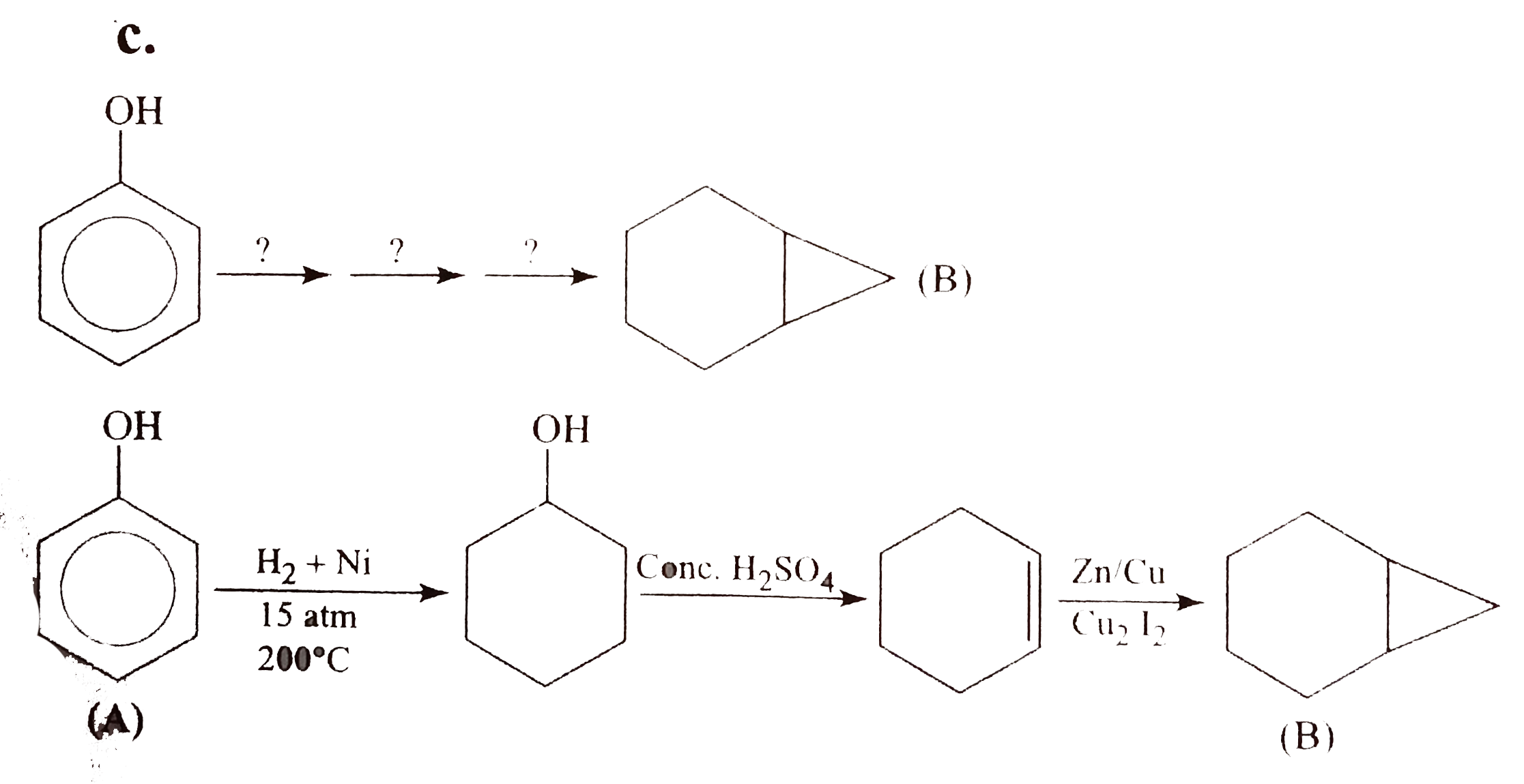
Draw the structure of aniline
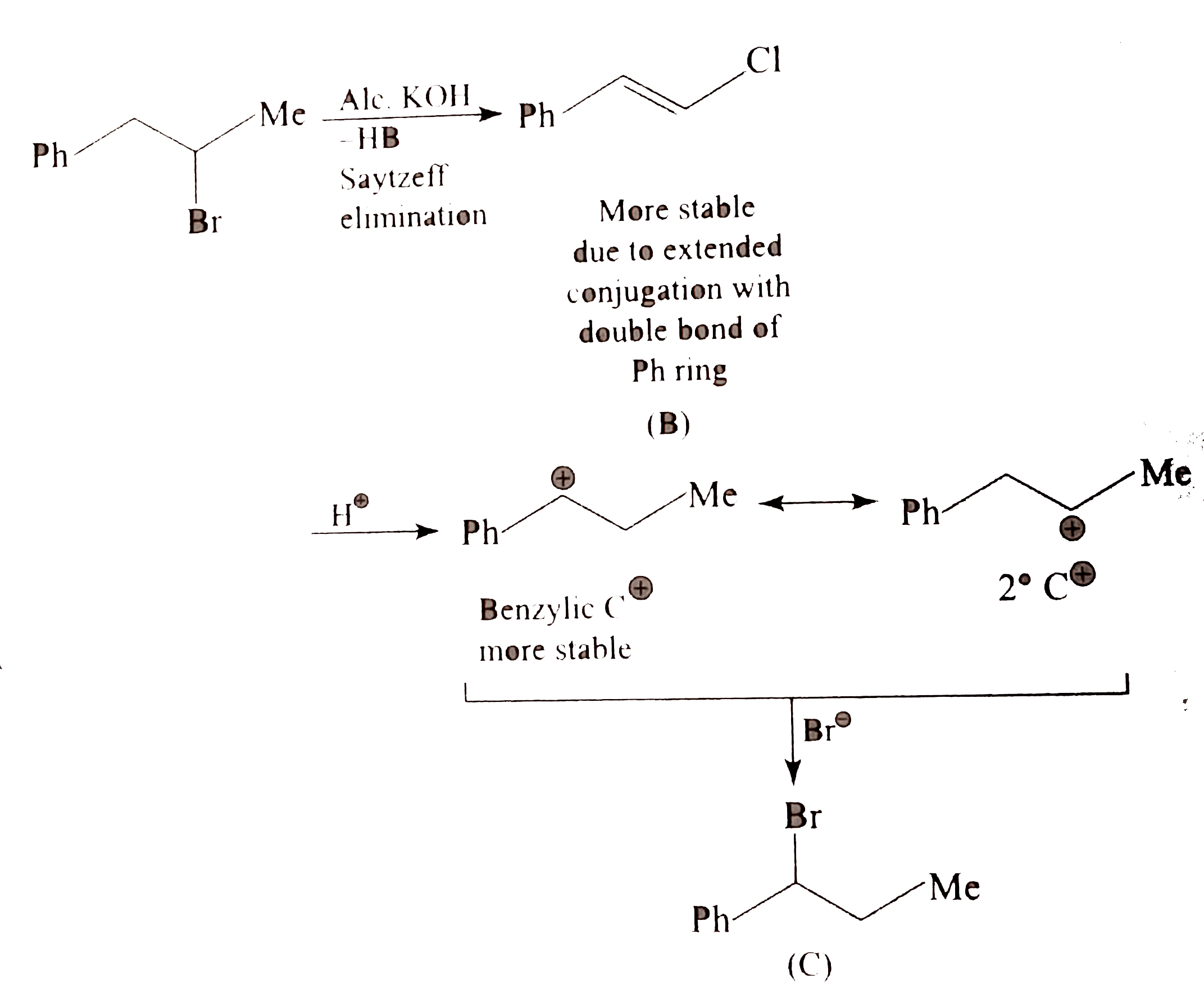
Draw the structure of aniline

Chapter 17 Amines and Amides Chemistry 114 with Divis at Franciscan

Aniline stock vector. Illustration of pigment, paracetamol 42026736
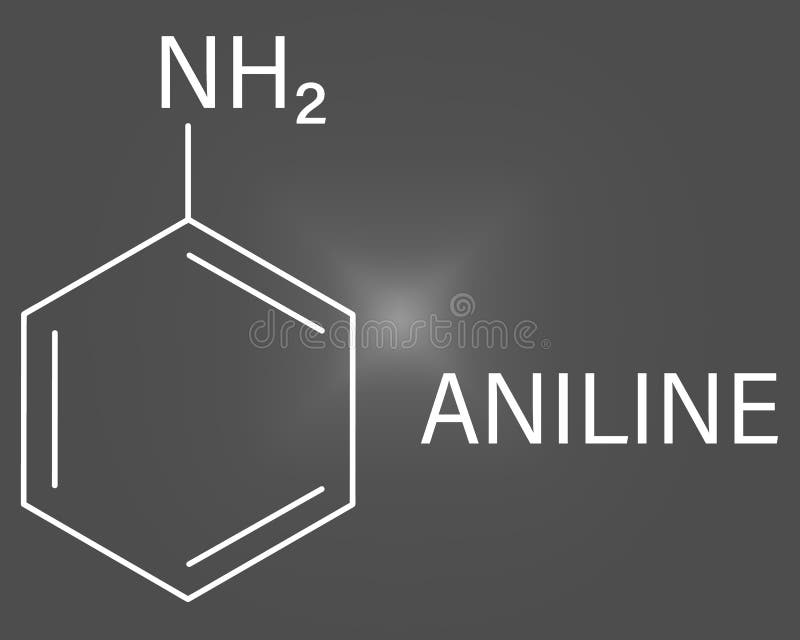
Aniline Molecule. Also Known As Phenylamine, Aminobenzene. Skeletal
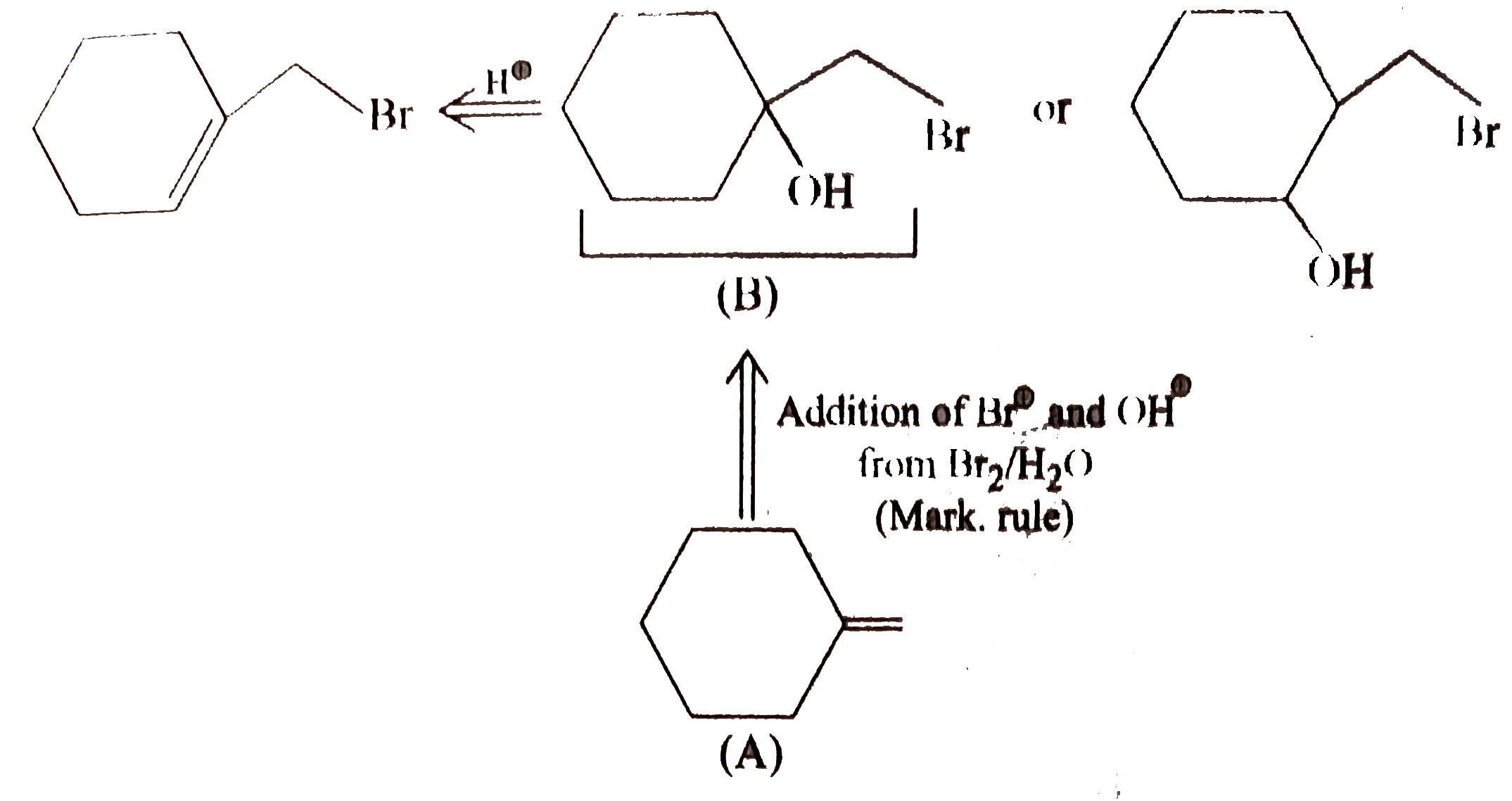
Draw the structure of aniline
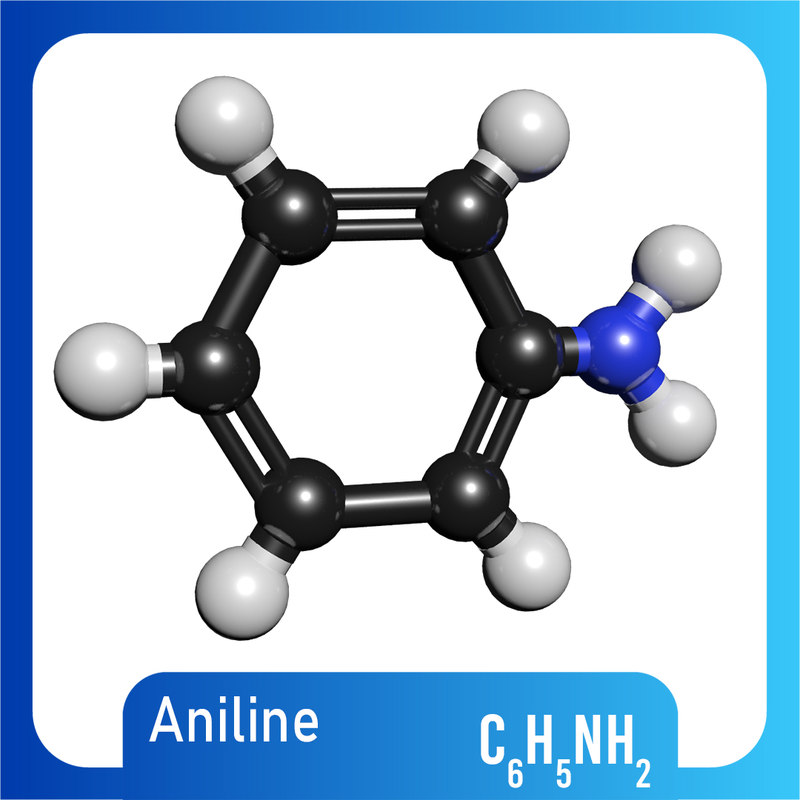
3D c6h5nh2 molecule aniline TurboSquid 1422230
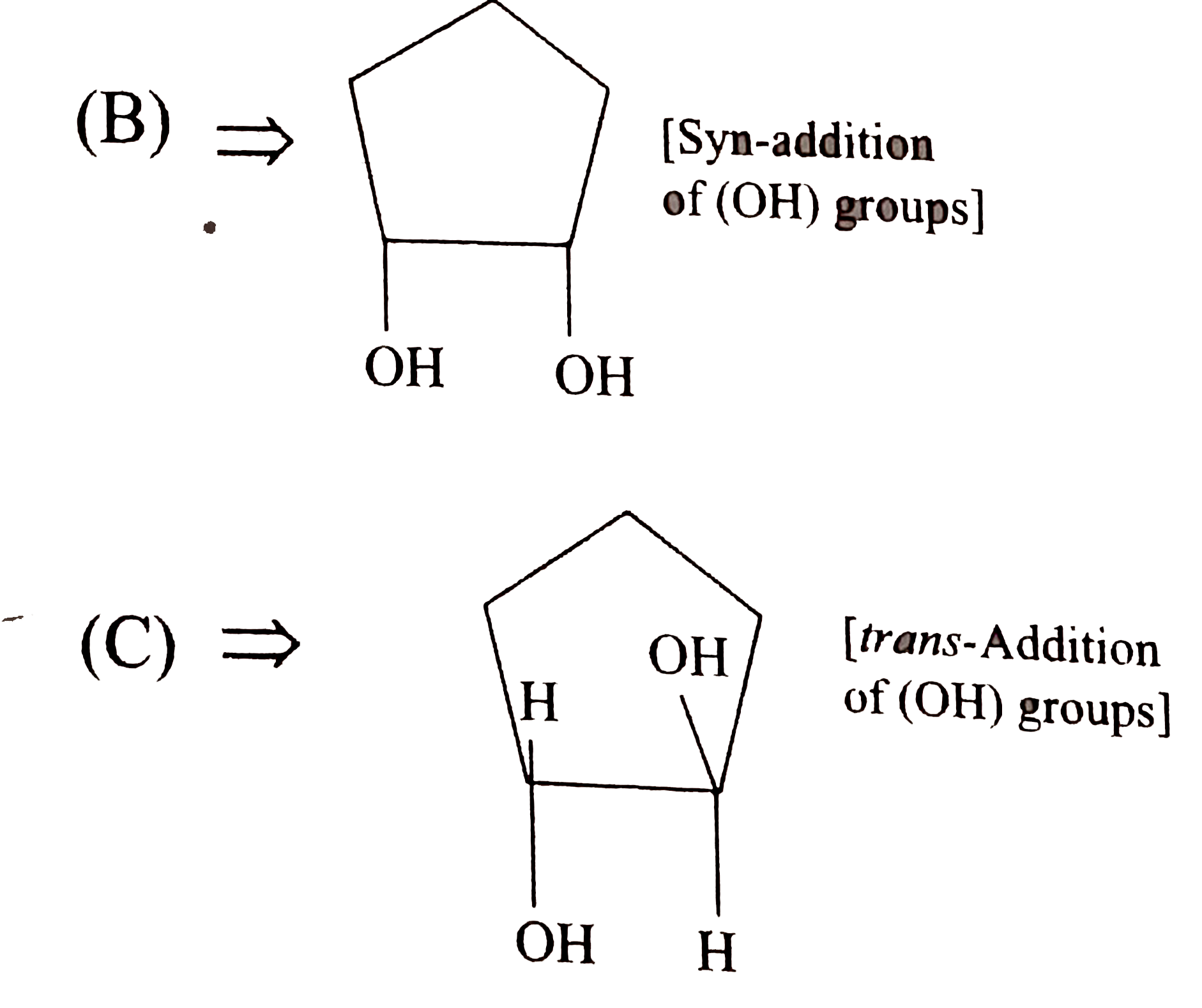
Draw the structure of aniline
Draw The Structure Of Aniline.
Web We Have Now Applied This Technique To An Evaluation Of The Structure Of The Aniline Molecule.
Draw The Structure For Each Compound And Classify The Amine As Primary, Secondary, Or Tertiary.
Web The Structure Of Phenylamine;
Related Post: