Draw The Structure Of Ch2O Including Lone Pairs
Draw The Structure Of Ch2O Including Lone Pairs - Draw the structure of ch 2 o, including lone pairs. C2cl4 draw the molecule by placing atoms on the grid and connecting them with bonds. Web it has a total of 12 valence electrons. Web chemistry chemistry questions and answers ch2o draw the molecule by placing atoms on the grid and connecting them with bonds. Carbon is a group iva element with four electrons in its last shell. Page contents show how to draw lewis structure for ch2o ch2o lewis structure is made up of one carbon (c), one oxygen (o), and two hydrogens (h). This problem has been solved! Include lone pairs of electrons. Web it is soluble in water and acetone. Draw the lewis structure for formaldehyde, ch, o. We cannot mark lone pairs on hydrogen atoms because thee are two electrons already in hydrogen's valence shell. Indicate which atom possesses the formal charge.… The structure comprises of electrons, like dots, drawn mostly in. For example, ch2o has a bent shape due to the lone pair of electrons on the oxygen atom, which contributes to. Determine the total number. Draw the structure for each of the following ions; Click on a bond to cycle through single, double, and triple. It has a boiling point of −19 °c and a melting point of −92 °c. Step by step solved in 3 steps with 3 images see solution check out a sample q&a here knowledge booster learn more about theories. Include. Web concept explainers question thumb_up 100% formaldehyde, ch 2 o, is used as an embalming agent. Include all lone pairs of electrons and nonbonding electrons. While it can be helpful initially to write the individual shared electrons, this approach quickly becomes awkward. The lewis structure of ch 2 o has two single bonds between the central carbon atom and two. Web lewis structure of ch2o contains a double bond between the carbon (c) & oxygen (o) atom and two single bonds between carbon (c) & hydrogen (h) atoms. Web science chemistry chemistry questions and answers draw the lewis structure for ch2o. Web science chemistry chemistry questions and answers a) for each of the following compounds, draw the lewis structure. Web. How many single bonds, double bonds, triple bonds, and lone pairs does it have? Determine the total number of electrons in the carbon, hydrogen, and oxygen valence shells. Lewis structure is one in which all lone pair and forma charge is shown shape is defined by… q: We cannot mark lone pairs on hydrogen atoms because thee are two electrons. The carbon atom (c) is at the center and it is surrounded by two hydrogen (h) and one oxygen atom (o). Mark remaining lone pairs on outside atoms. Draw the lewis structure of the missing reactant. The oxygen atom has 2 lone pairs. Web here’s how you can easily draw the ch 2 o lewis structure step by step: Click on a bond to cycle through single, double, and triple. Draw the structure of ch 2 o, including lone pairs. Web chemistry chemistry questions and answers ch2o draw the molecule by placing atoms on the grid and connecting them with bonds. Step by step solved in 3 steps with 3 images see solution check out a sample q&a here. The oxygen atom also has two lone pairs of electrons. Web lewis structure of ch2o contains a double bond between the carbon (c) & oxygen (o) atom and two single bonds between carbon (c) & hydrogen (h) atoms. The oxygen atom has 2 lone pairs. In pure form, it smells pungent. #1 draw a rough skeleton structure #2 mention lone. Web it has a total of 12 valence electrons. Include all lone pairs of electrons and nonbonding electrons. Draw the structure of ch 2 o, including lone pairs. Co2 сн,он ch2o hcooh ch4 b) in which of these compounds is the extent of oxidation the greatest? It has a boiling point of −19 °c and a melting point of −92. Web here’s how you can easily draw the ch 2 o lewis structure step by step: Web chemistry chemistry questions and answers ch2o draw the molecule by placing atoms on the grid and connecting them with bonds. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4. The carbon atom (c) is at the center and it is surrounded by two hydrogen (h) and one oxygen atom (o). Step by step solved in 3 steps with 3 images see solution check out a sample q&a here knowledge booster learn more about theories. Select draw rings more ch select the intermolecular forces present between ch, o molecules. Indicate which atom possesses the formal charge.… Web concept explainers question thumb_up 100% formaldehyde, ch 2 o, is used as an embalming agent. Include all lone pairs of electrons and nonbonding electrons. #1 draw a rough skeleton structure #2 mention lone pairs on the atoms #3 if needed, mention formal charges on the atoms #4 minimize formal charges by converting lone pairs of the atoms, and try to get a stable lewis structure #5 repeat step 4 again if needed, until all. Web draw the structure of ch2o including lone pairs. Web it is a simple organic compound with the chemical formula ch2o. Web for a molecular formula of ch2o, we would have 12 valence electrons to distribute around the molecule, or 6 pairs of electrons. Web there are important things to consider drawing the lewis structure of hcho because there are seven elements in that molecule and it is bit complex molecule too. The oxygen atom has 2 lone pairs. Web draw the structure of ch2o, including lone pairs. Draw the structure for each of the following ions; Click on a bond to cycle through single, double, and triple. Web the lewis structure of ch2o provides insight into the molecule’s shape and bonding pattern, which in turn affects its physical properties.
Draw the Structure of Ch2o Including Lone Pairs

CH2O Lewis Structure Lewis Dot Structure for CH2O Methanal or

CH2O Lewis Structure, Molecular Geometry, and Hybridization

CH2O Lewis Structure, Molecular Geometry, and Hybridization

lewis structure of ch2o

CH2O Molecular Geometry,Shape and Bond Angles (Formaldehyde)

Bonding in the CH2O Molecule YouTube

Formaldehyde Formula CH2O Name, Structure, Uses, Compound
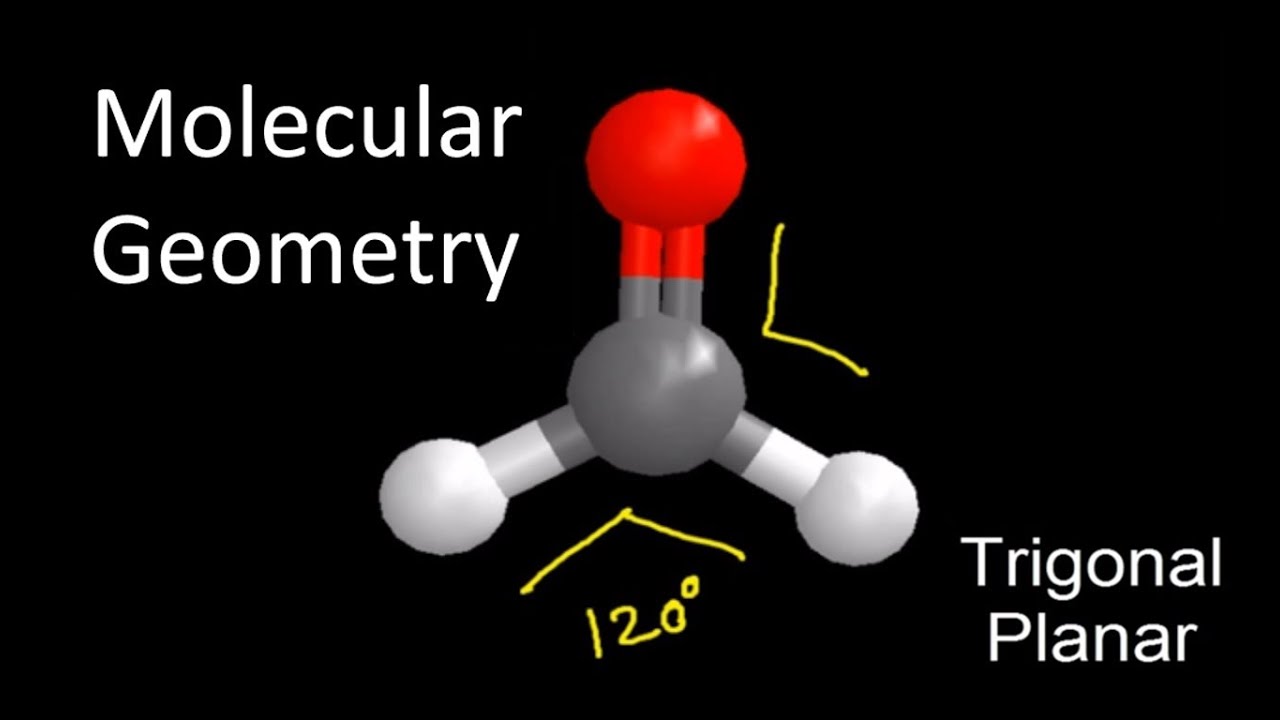
CH2O Molecular Geometry / Shape and Bond Angles YouTube

How to Draw the Lewis Dot Structure for CH2O Formaldehyde YouTube
We Cannot Mark Lone Pairs On Hydrogen Atoms Because Thee Are Two Electrons Already In Hydrogen's Valence Shell.
There Are No Lone Pair Of Electrons On The Central Carbon Atom, But The Oxygen Atom Has Two Lone Electron Pairs.
Include All Lone Pairs Of Electrons And Nonbonding Electrons.
It Has A Boiling Point Of −19 °C And A Melting Point Of −92 °C.
Related Post: