Drawing Carbon Atom
Drawing Carbon Atom - It belongs to group 14 of the periodic table. Each hydrogen is one valence electron, but we have two of them, so 1 times 2. 13k views 11 years ago. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. Web rule 1 carbon atoms aren’t usually shown. It has symbol c and atomic number 6. The butane skeleton has four points to represent the 4 carbon atoms notice that the first and last carbon atoms are represented. Web points to remember when drawing a skeletal or line diagram: Calculate the total number of valence electrons. Web step 1: Web rule 1 carbon atoms aren’t usually shown. Lewis symbols we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these. Each hydrogen is one valence electron, but we have two of them, so 1 times 2. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. The butane skeleton has four points to represent the 4 carbon atoms notice that the first and last carbon. It belongs to group 14 of the periodic table. Add enough electrons (dots) to the outer atoms to. Web the chemdoodle web components library is a pure javascript chemical graphics and cheminformatics library derived from the chemdoodle application and produced by ichemlabs. Lewis symbols we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Web i. (valence electrons are the number of electrons present in the outermost shell of an atom). Web carbon (from latin carbo 'coal') is a chemical element; This is even more important when you start to have branched chains of carbon atoms. Web we have one atom of carbon, so that's four valence electrons. Web drawing a carbon atom. Web the chemdoodle web components library is a pure javascript chemical graphics and cheminformatics library derived from the chemdoodle application and produced by ichemlabs. Common errors when drawing multiple bonds. 13k views 11 years ago. Occasionally, a carbon atom might be indicated for emphasis or clarity. Each hydrogen is one valence electron, but we have two of them, so 1. Carbon makes up about 0.025 percent of earth's crust. Web carbon (from latin carbo 'coal') is a chemical element; And each oxygen is six, and we have one of them, so six. Instead, a carbon atom is assumed to be at each intersection of two lines (bonds) and at the end of each line. Web a carbon atom is present. Web in this video we'll look at the atomic structure and bohr model for the carbon atom (c). Web step 1: (valence electrons are the number of electrons present in the outermost shell of an atom). And each oxygen is six, and we have one of them, so six. Carbon has 2 electrons in its first shell and 4 in. Carbon makes up about 0.025 percent of earth's crust. Calculate the total number of valence electrons. Here, the given molecule is co2 (carbon dioxide). Add enough electrons (dots) to the outer atoms to. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Lewis symbols we use lewis symbols to describe valence electron configurations of atoms and monatomic ions. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web drawing a carbon atom. Each hydrogen is one valence electron, but we have two of them, so 1 times 2. Web the chemdoodle web. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. Web in this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely lewis symbols and lewis structures. Draw the lewis dot structure for the hydrogen atom. Common errors. In order to draw the lewis structure of co2, first of all you have to find the total number of valence electrons present in the co2 molecule. Hydrogens that are attached to elements other than carbon are shown. Carbon has 2 electrons in its first shell and 4 in its second shell. Common errors when drawing multiple bonds. Web in this video we'll look at the atomic structure and bohr model for the carbon atom (c). It belongs to group 14 of the periodic table. Web points to remember when drawing a skeletal or line diagram: Web draw a skeleton with each carbon atom represented using a point: Web i quickly take you through how to draw the lewis structure of co2 (carbon dioxide). It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. Each hydrogen is one valence electron, but we have two of them, so 1 times 2. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Web the chemdoodle web components library is a pure javascript chemical graphics and cheminformatics library derived from the chemdoodle application and produced by ichemlabs. (valence electrons are the number of electrons present in the outermost shell of an atom). Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Two oxygen atoms are present at the terminals, where they share electrons and form bonds with the central carbon atom.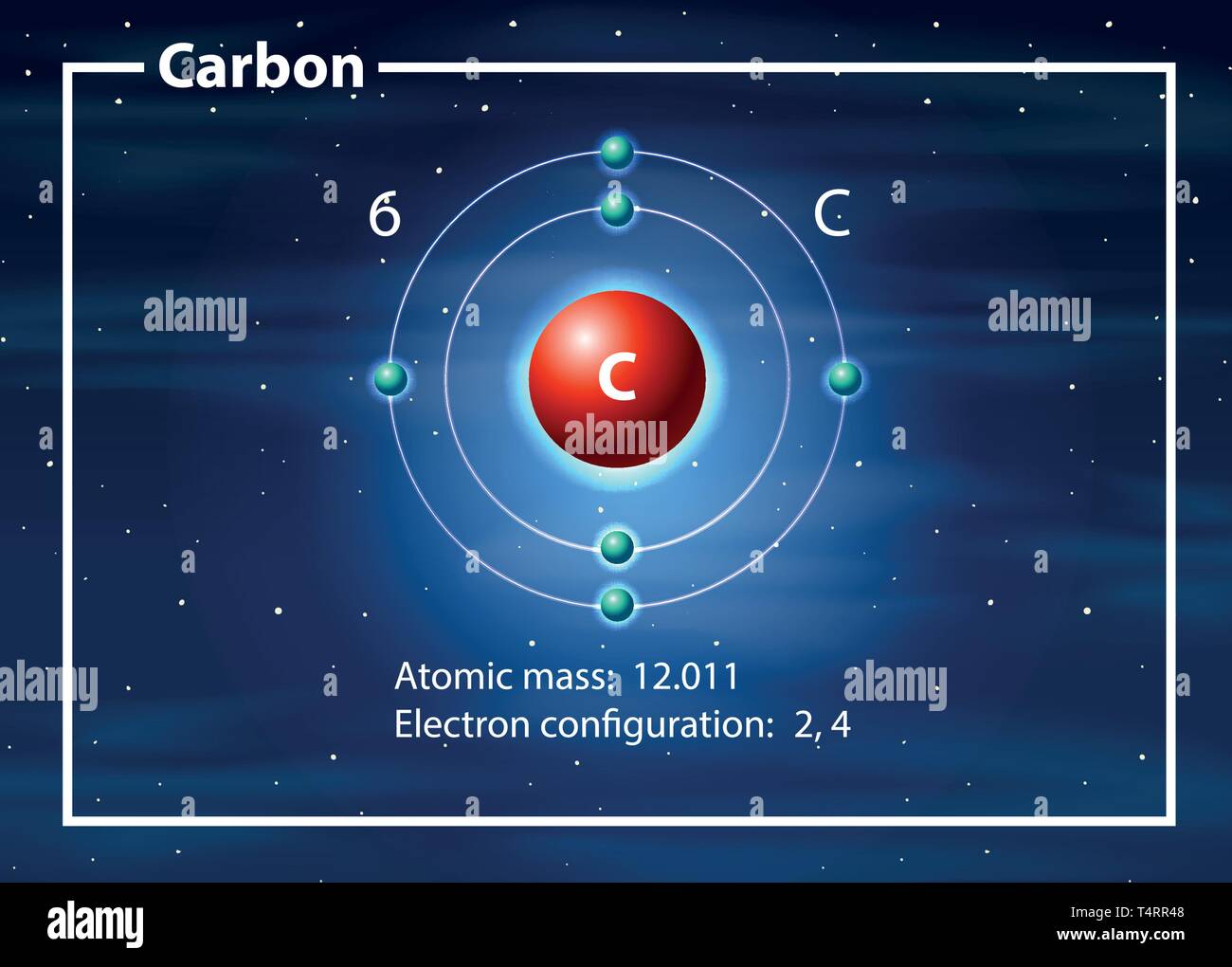
Carbon atom diagram hires stock photography and images Alamy

Carbon Atom Structure 3d
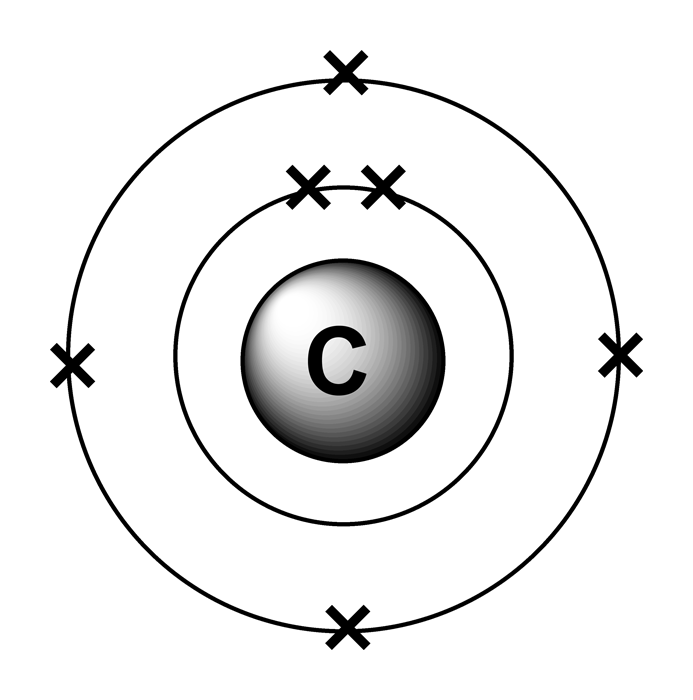
Electron configurations
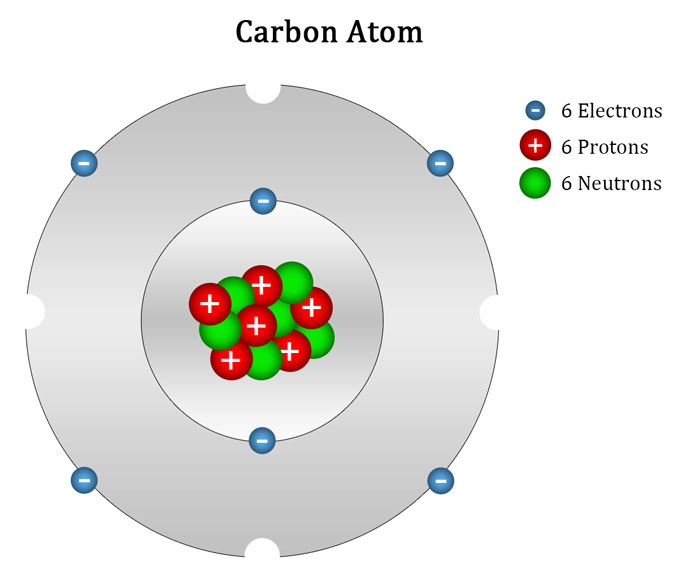
Carbon Atom Ascension Glossary
Diagram Of A Carbon Atom Stock Illustration Getty Images
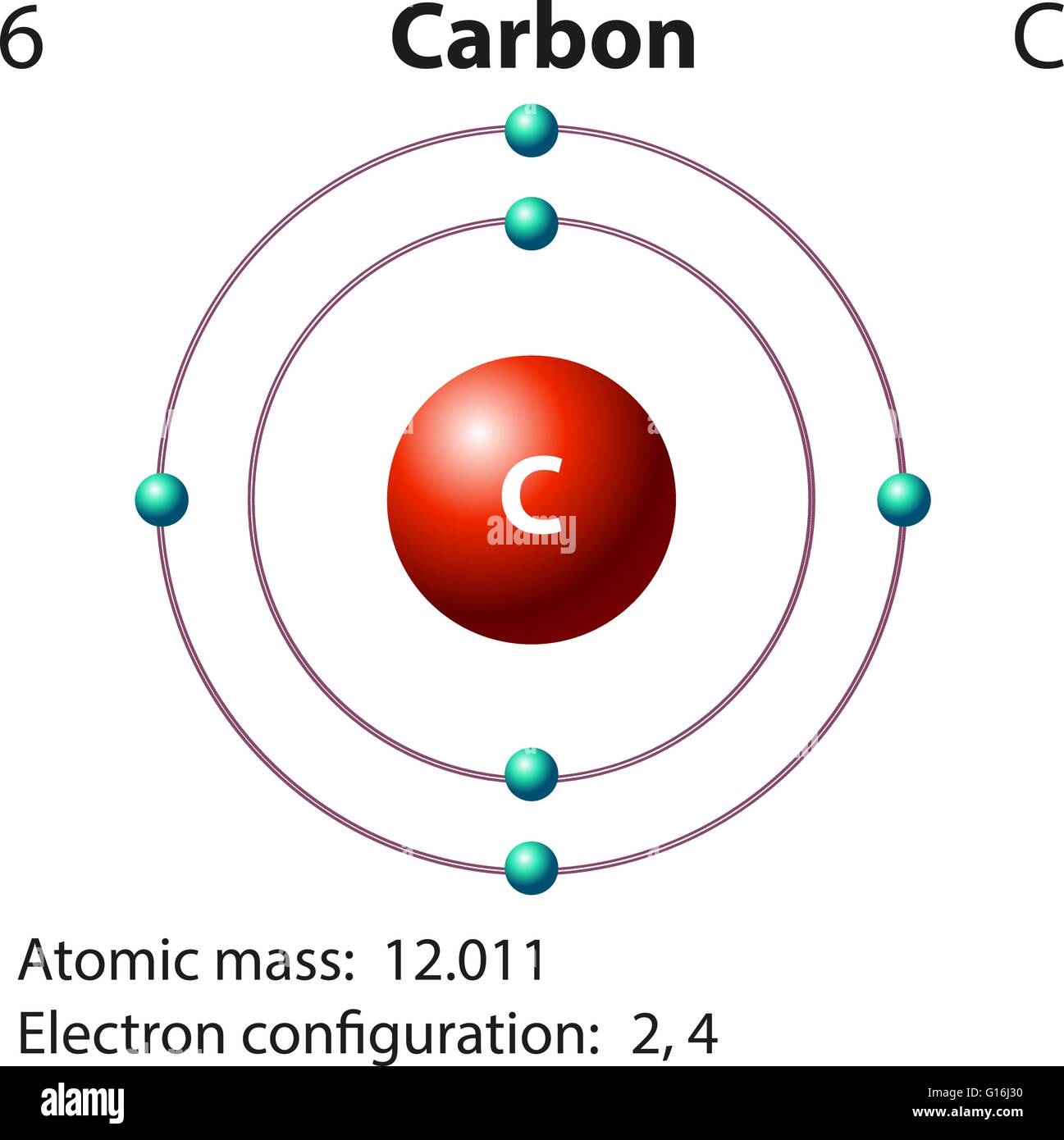
Carbon atom diagram hires stock photography and images Alamy
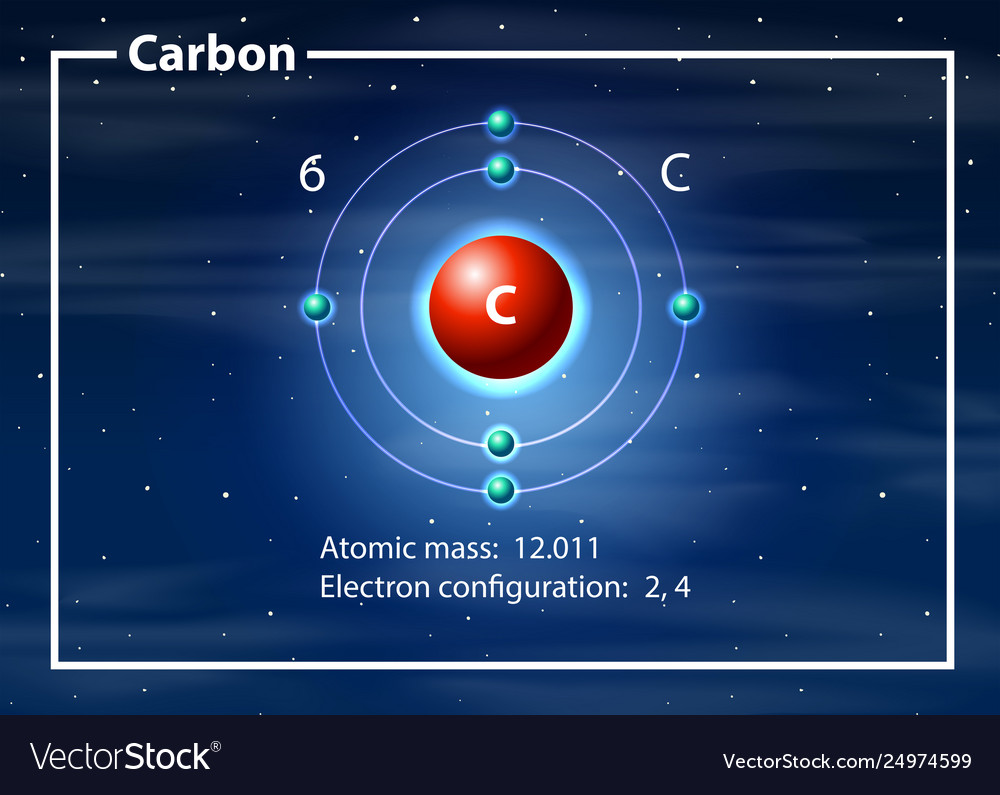
Carbon atom diagram concept Royalty Free Vector Image

Carbon atomic structure (437243) Illustrations Design Bundles
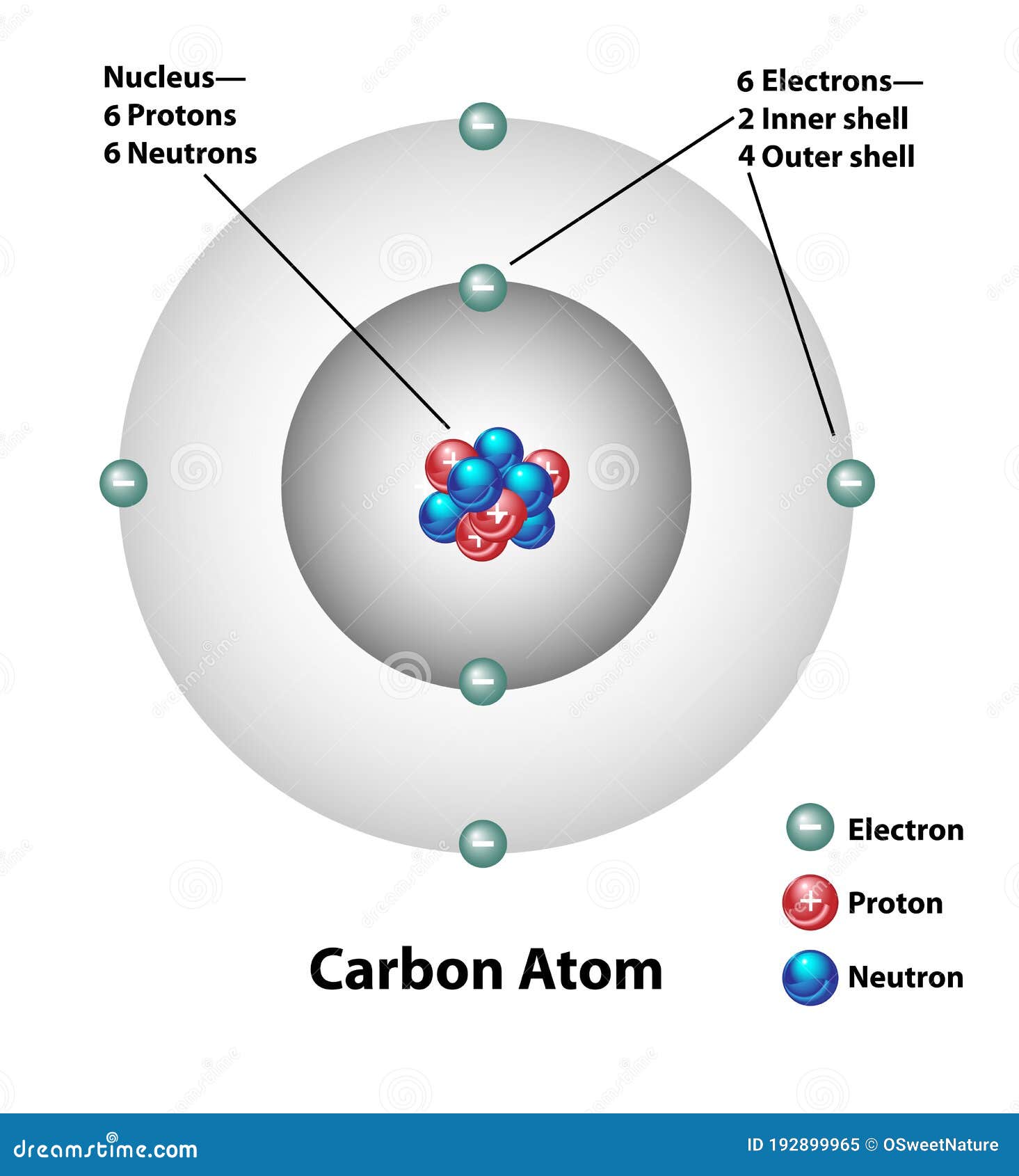
Carbon Atom Molecular Structure Labels Stock Vector Illustration of
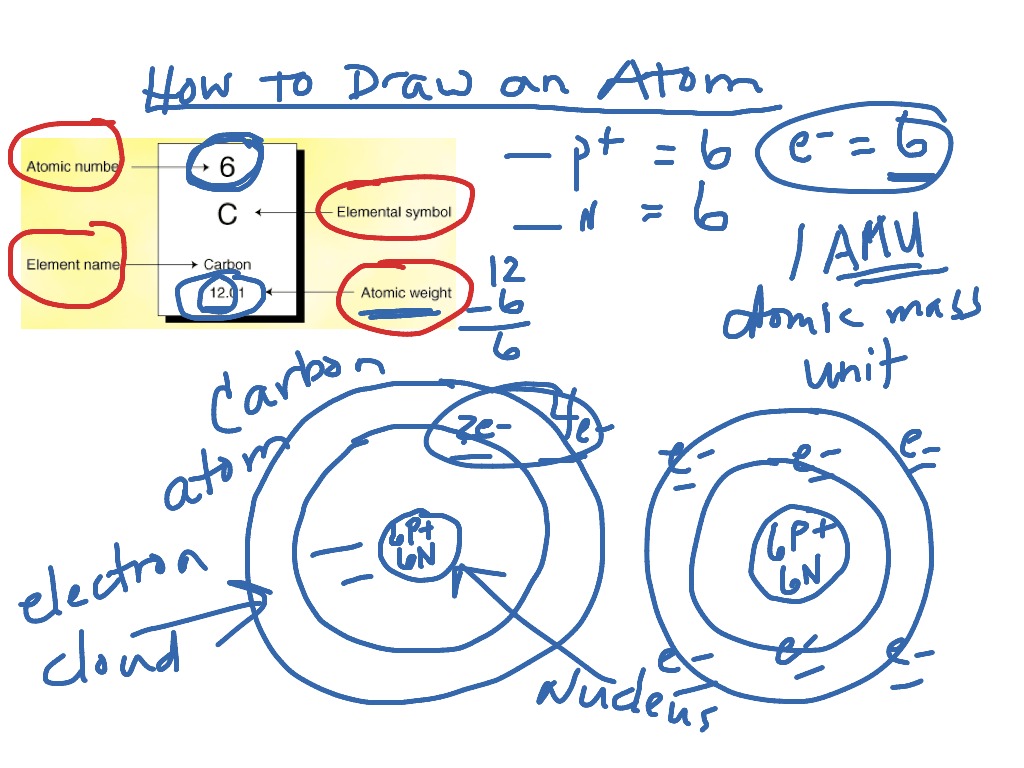
How to draw an atom of carbon Science ShowMe
Draw The Lewis Dot Structure For The Hydrogen Atom.
Connect The Atoms To Each Other With Single Bonds To Form A “Skeleton Structure.” Be Sure That You Follow Rule 1.
Here, The Given Molecule Is Co2 (Carbon Dioxide).
Web Rule 1 Carbon Atoms Aren’t Usually Shown.
Related Post:
