Drawing Of Fluorine
Drawing Of Fluorine - Fluorine has 2 electrons in its first shell and 7 in its second.check me out:. Web choose from drawing of fluorine stock illustrations from istock. Figure 2 2 contrast the bohr diagrams for lithium, fluorine and aluminum atoms. Electron configuration can be done in two ways. Connect the atoms to each other with single bonds to form a “skeleton structure.”. The electron configuration of fluorine is [ he] 2s 2 2p 5, if the electron arrangement is through orbitals. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. So fluorine needs one electron to fill the octet structure. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. The electron configuration of fluorine is 1s 2 2s 2 2p 5. Fundraising has been sharply lower in 2023, with brookfield’s recent raise representing almost. Champions league round of 16 teams. Web the arrangement of electrons in fluorine in specific rules in different orbits and orbitals is called the electron configuration of fluorine. Fluorine is situated in group 17th and has an atomic number of 9. The electron configuration of fluorine is. Napoli vs barcelona, inter vs atlético de madrid, lepzig vs real madrid. The atomic number of fluorine represents the total number of electrons of fluorine. Now in the next step, start. Web infrastructure investing today spans from toll roads to energy assets and semiconductor factories. Web for example, consider fluorine and sulfur. Web fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. Napoli vs barcelona, inter vs atlético de madrid, lepzig vs real madrid. #1 write protons, neutrons, and electrons of fluorine atom Connect the atoms to each other with single bonds to form a “skeleton structure.”. Number of steps can be changed. The atomic number of fluorine represents the total number of electrons of fluorine. Web periodic table of videos created by video journalist brady haran working with chemists at the university of nottingham. Now in the next step, start. Web how to draw the lewis dot structure for f2 : Web infrastructure investing today spans from toll roads to energy assets. Web fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. #1 write protons, neutrons, and electrons of fluorine atom It accept electron from donor atom to gain noble gas like stability. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.. The same scoreline followed 24 hours later as aston villa failed to beat. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Fluorine has 2 electrons in its first shell and 7 in its second.check me out:. However those all steps are mentioned and explained in detail in this.. The atomic number of fluorine represents the total number of electrons of fluorine. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Web in this video we'll look at the atomic structure and bohr model for the fluorine atom (f). Web to write the orbital diagram for the fluorine atom (f) first we need to. In the bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Science history images / alamy stock photo. Web choose from drawing of fluorine stock illustrations from istock. The electron configuration of fluorine is 1s 2 2s 2 2p 5. The shell closest to the nucleus is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and. #1 write protons, neutrons, and electrons of fluorine atom #2 draw nucleus of fluorine atom #3 draw 1 st electron shell #4 draw 2 nd electron shell let’s break down each step in detail. Because f 2 is a simple molecule, it is extremely easy to draw the lewis structure. The first shell of fluorine has 2 electrons and the outer shell or valence shell of fluorine has 7 electrons, hence, the number of valence. The number of valence electrons available for fluorine atoms is 7. Web fluorine is the most electronegative element on the periodic table, which means that it is a very. They face off in a reprise. Now in the next step, start. Web how to draw the lewis dot structure for f2 : Champions league round of 16 teams. Napoli vs barcelona, inter vs atlético de madrid, lepzig vs real madrid. Web the arrangement of electrons in fluorine in specific rules in different orbits and orbitals is called the electron configuration of fluorine. Web to draw fluorine lewis dot structure, we have to count valence electrons of fluorine that is 7 which are written as dots around “f”. Web steps find electrons. Number of steps can be changed according the complexity of the molecule or ion. Web choose from drawing of fluorine stock illustrations from istock. Web champions league round of 16 draw: Web fluorine is the most electronegative element on the periodic table, which means that it is a very. Fluorine ion lewis dot structure fluorine is ‘group 17’ element in periodic table. When we draw a lewis structure, guidelines are given. It accept electron from donor atom to gain noble gas like stability. As the most electronegative element, it is extremely reactive: Web fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions.
Fluorine F (Element 9) of Periodic Table Elements Flash Cards
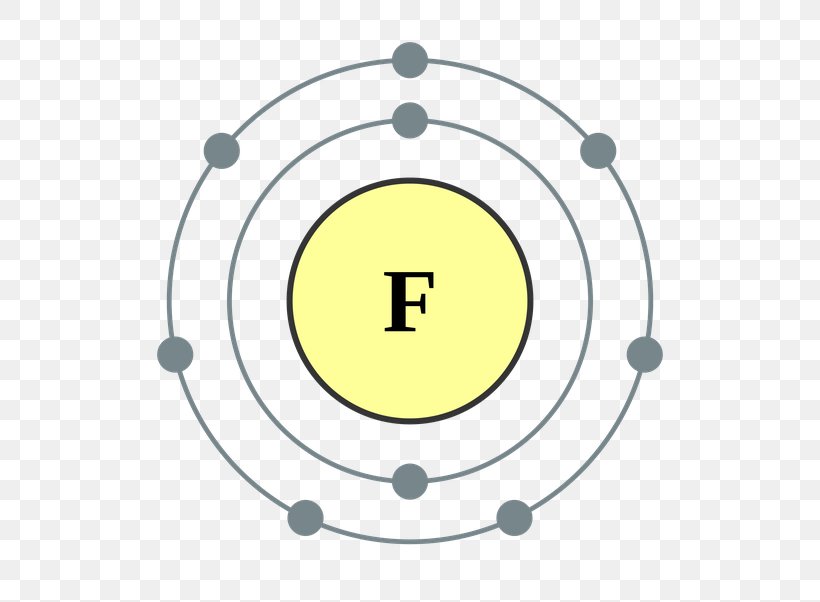
Electron Shell Fluorine Atom Periodic Table Chemical Element, PNG
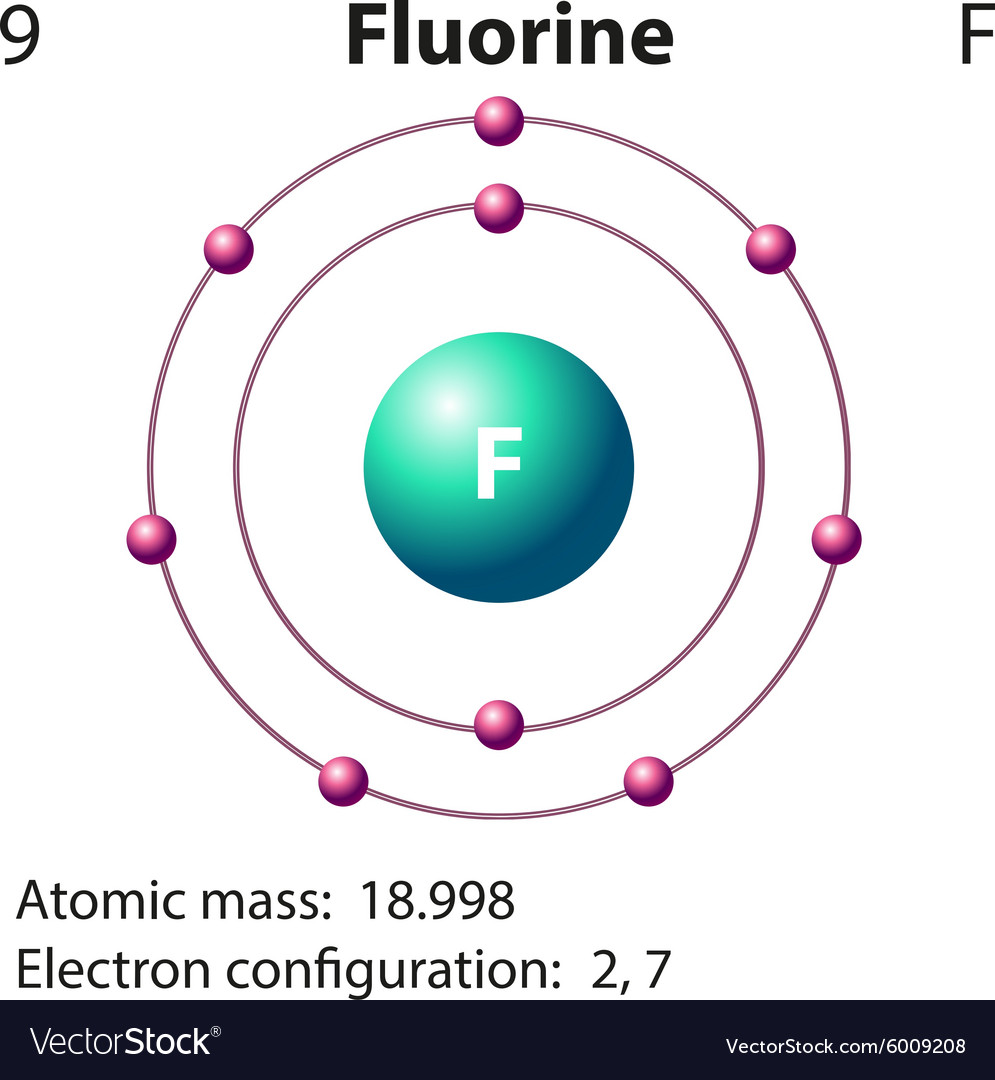
Diagram representation of the element fluorine Vector Image

Fluorine chemical element Royalty Free Vector Image

Fluorine, atomic structure Stock Image C013/1510 Science Photo

Fluorine Diagram
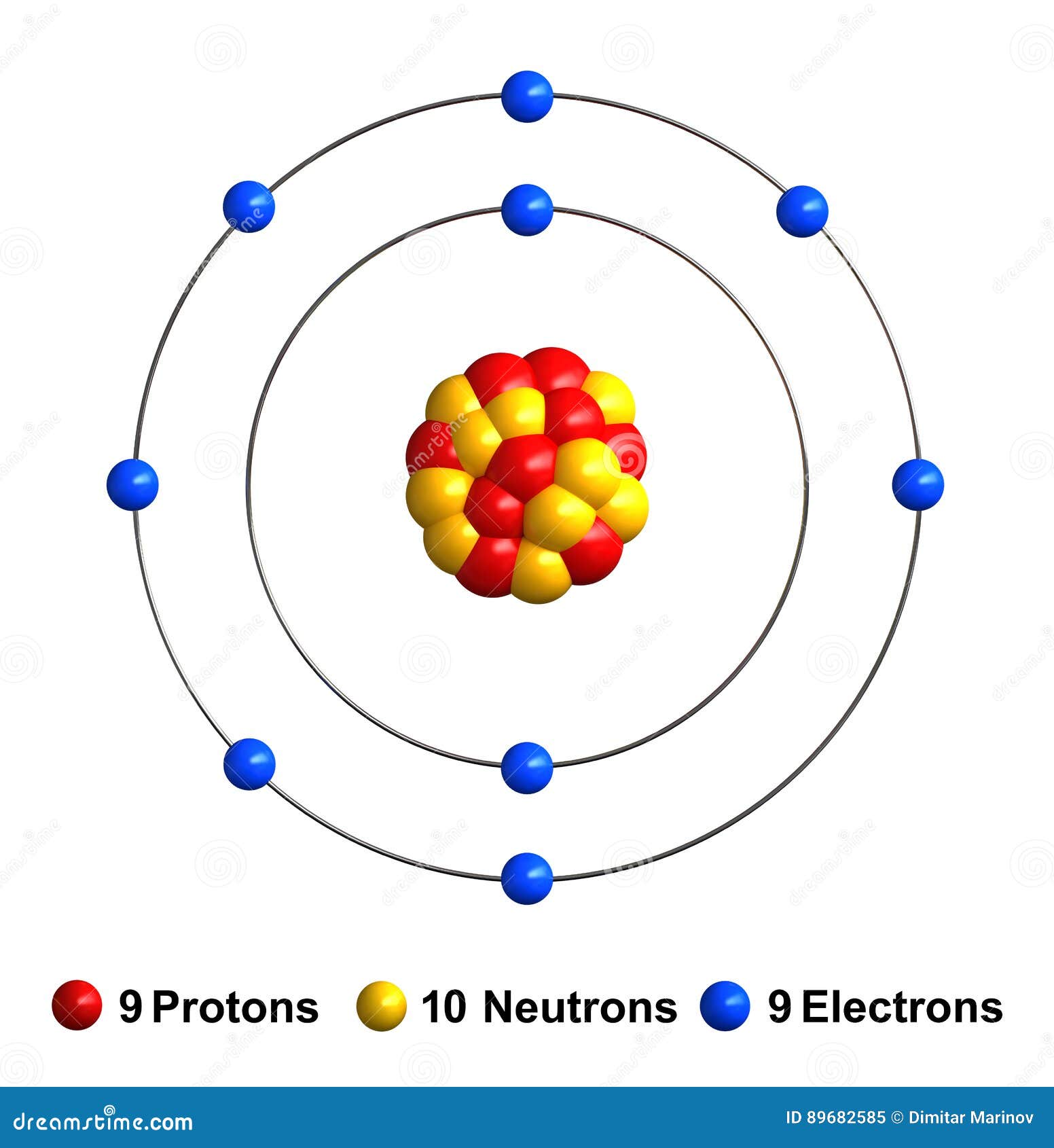
Fluorine stock illustration. Illustration of protons 89682585

fluorine orbital diagram Herbalned
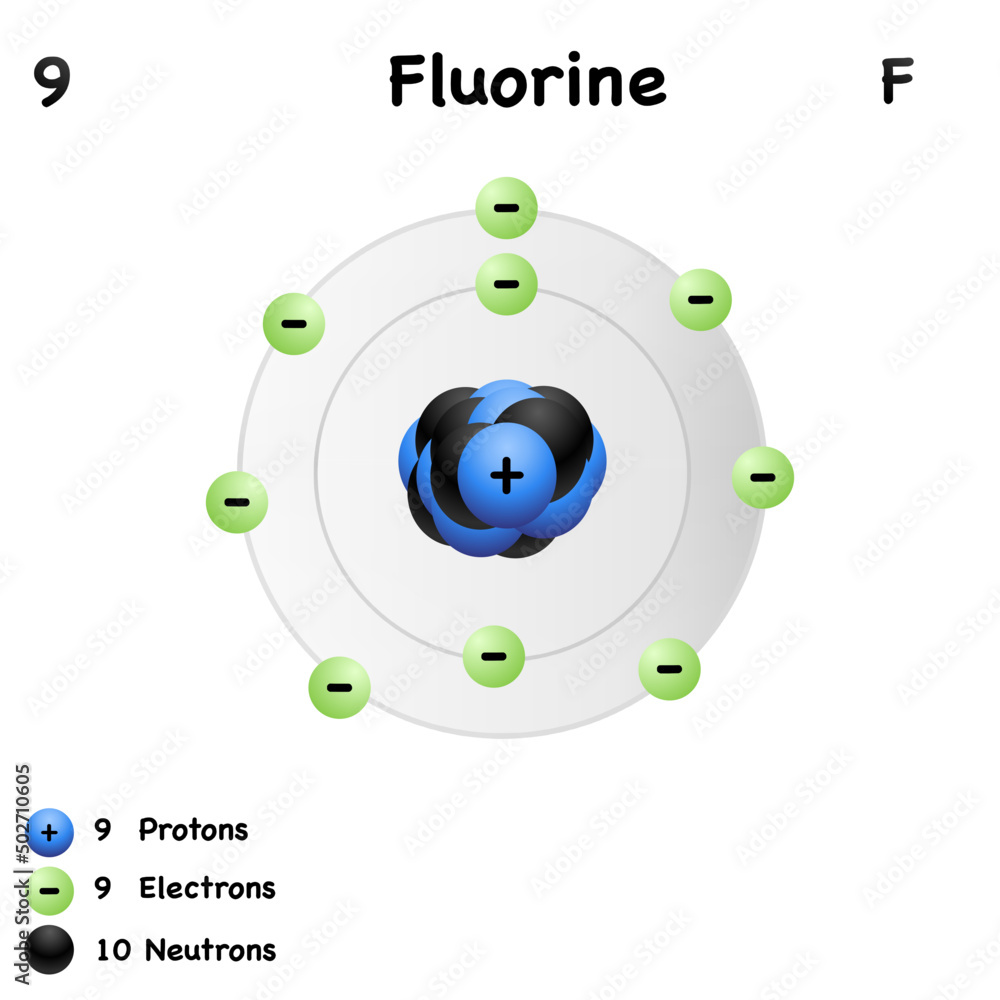
Fluorine element with symbol C and atomic number 9.Isolated molecular

Chemical Elements atomic_structure_of_fluorine_color Classroom Clipart
And Then There Is Hydrogen.
Electron Configuration Can Be Done In Two Ways.
Web For Example, Consider Fluorine And Sulfur.
Illustration From 1887 By E.
Related Post: