Drawing The Reaction Energy Diagram Of A Catalyzed Reaction
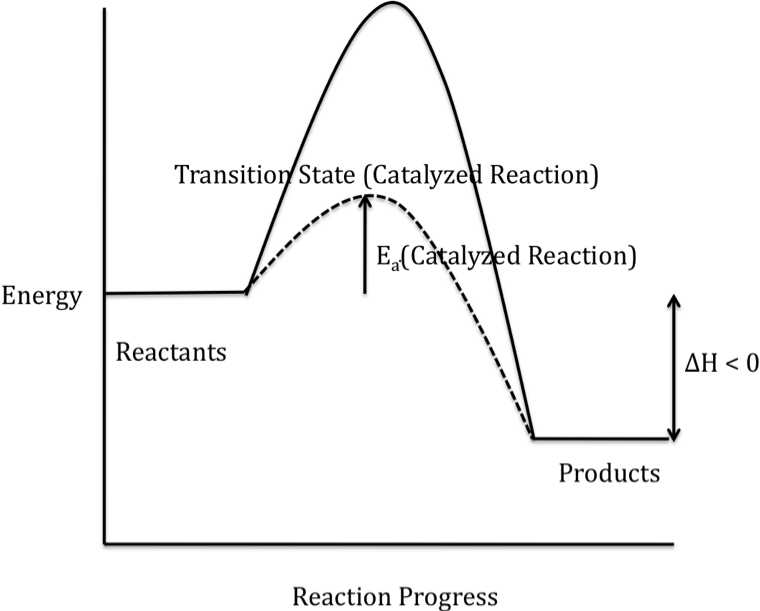
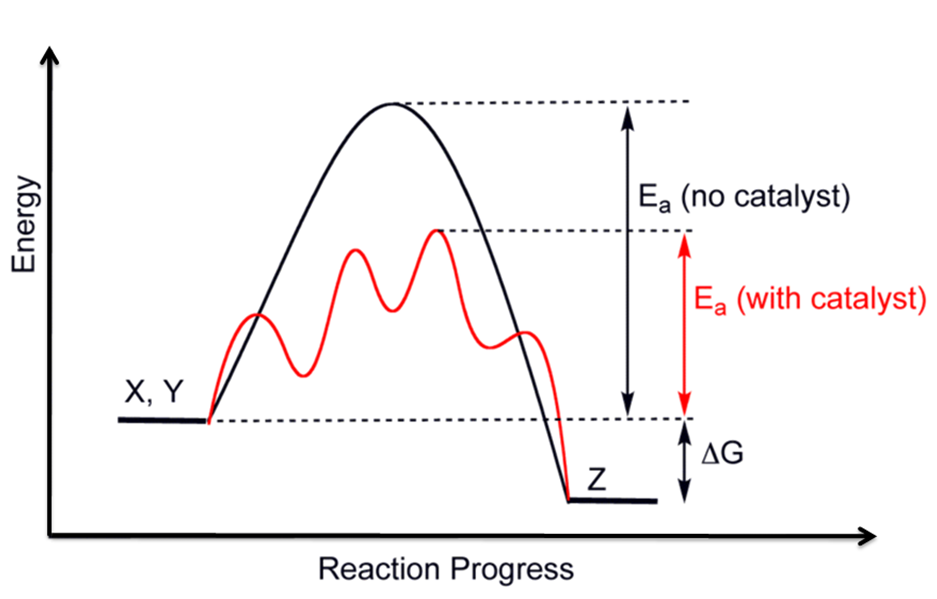
Drawing The Reaction Energy Diagram Of A Catalyzed Reaction - It also shows the effect of a catalyst on the forward and reverse activation energy. A catalyst like iodine can be used to provide an alternate pathway for the reaction with a much lower activation energy of approximately 118 kj/mol (figures 17.15 “catalyzed. Web draw reaction energy diagrams from the thermodynamic and kinetic data/information. They are, as we would expect, identical in that respect. Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. The reaction with catalyst is indicated with a blue line, and the uncatalyzed reaction is indicated with a red line. Use a reaction energy diagram to discuss transition states, ea, intermediates & rate determining step. Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed. Web drawing the reaction energy diagram of a catalyzed reaction ap chemistry skills practice 1. Web draw reaction energy diagrams from the thermodynamic and kinetic data/information. Show solution check your learning Draw the transition state of a reaction. The catalyst does not affect the energy of the reactants or products (and thus does not affect δ e ). Using reaction diagrams to compare catalyzed reactions the two reaction diagrams here represent the same reaction: The catalyst does not affect the energy of the reactants or. Web identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: The reaction with catalyst is indicated with a blue line, and the uncatalyzed reaction is indicated with a red line. Web the two reaction diagrams below represent the same reaction:. Determine the energies of the reactants and products and the reaction enthalpy from the labeled diagram above,. Web reaction diagrams for catalyzed reactions. The only effect of the catalyst is to lower the activation energy of the reaction. Web the two reaction diagrams below represent the same reaction: Web drawing the reaction energy diagram of a catalyzed reaction ap chemistry. Web in an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. The two reaction diagrams here represent the same reaction: A catalyst increases the rate of a reaction by altering the mechanism,. The two reaction diagrams here represent the same reaction: The only effect of the catalyst is to lower the activation energy of the reaction. They are, as we would expect, identical in that respect. One without a catalyst and one with a catalyst. Web draw reaction energy diagrams from the thermodynamic and kinetic data/information. The reaction with catalyst is indicated with a blue line, and the uncatalyzed reaction is indicated with a red line. The only effect of the catalyst is to lower the activation energy of the reaction. In this figure, two graphs are shown. Analyzing the potential energy diagram of a regular/uncatalyzed and a catalyzed (adding a catalyst) reaction. Web draw reaction. Show solution check your learning The only effect of the catalyst is to lower the activation energy of the reaction. The two reaction diagrams here represent the same reaction: The catalyst does not affect the energy of the reactants or products (and thus does not affect δ e ). Web draw reaction energy diagrams from the thermodynamic and kinetic data/information. The only effect of the catalyst is to lower the activation energy of the reaction. A catalyst like iodine can be used to provide an alternate pathway for the reaction with a much lower activation energy of approximately 118 kj/mol (figures 17.15 “catalyzed. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works. How do molecules have to be arranged and how much energy do they have to collide with? Web figure 14.7.1 14.7. Web the two reaction diagrams below represent the same reaction: A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction.. The two reaction diagrams here represent the same reaction: The catalyst does not affect the energy of the reactants or. A catalyst increases the rate of a reaction by altering the mechanism, allowing the reaction to proceed via a pathway with lower activation energy than for the uncatalyzed reaction. Web the following diagram shows an energy diagram for the reaction. The two reaction diagrams here represent the same reaction: Lowering the activation energy of a reaction by a catalyst. Because the sketches are only qualitative, the energies in them don't have to be exact. The activation energy is also lower, which means that less energy is required to initiate. One without a catalyst and one with a catalyst. Web drawing the reaction energy diagram of a catalyzed reaction ap chemistry skills practice 1. Determine the energies of the reactants and products and the reaction enthalpy from the labeled diagram above,. Estimate the activation energy for each process, and identify which one involves a catalyst. Solution a catalyst does not affect the energy of reactant or product, so those aspects of the diagrams can be ignored; Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed. What does the orange line represent? You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. Assume the uncatalyzed reaction is endothermic note: One without a catalyst and one with a catalyst. One without a catalyst and one with a catalyst. Web this chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.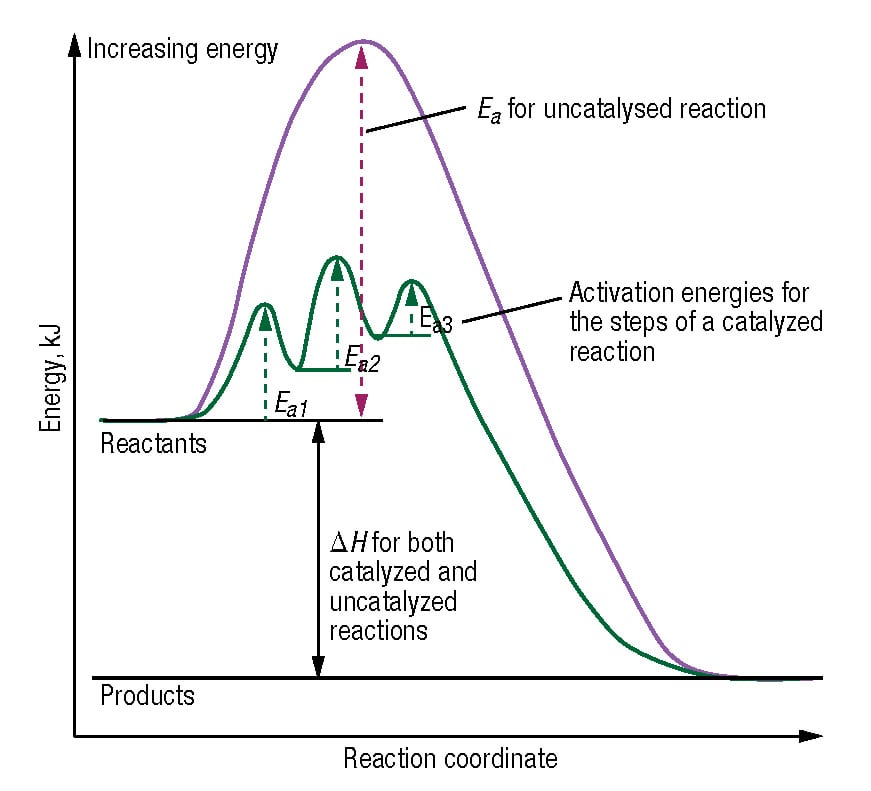
Catalysis Fundamentals Chemical Engineering Page 1
Section 184 Catalysis
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry
Effect of catalyst on energy diagram profile. Download Scientific Diagram
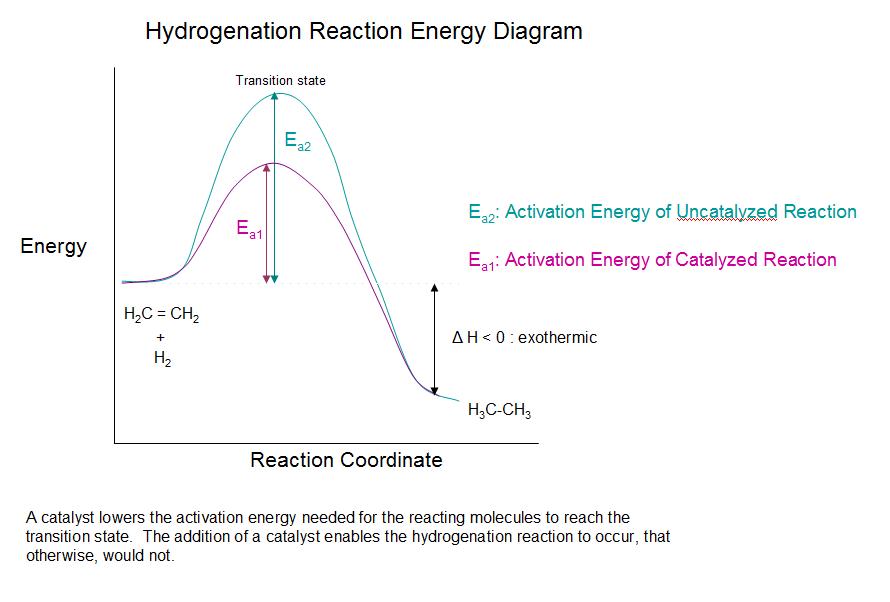
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts
Catalysts in 21st Century Energy

Energy Diagram Catalyzed Vs Uncatalyzed Reaction

Energy Diagram Catalyzed Vs Uncatalyzed Reaction
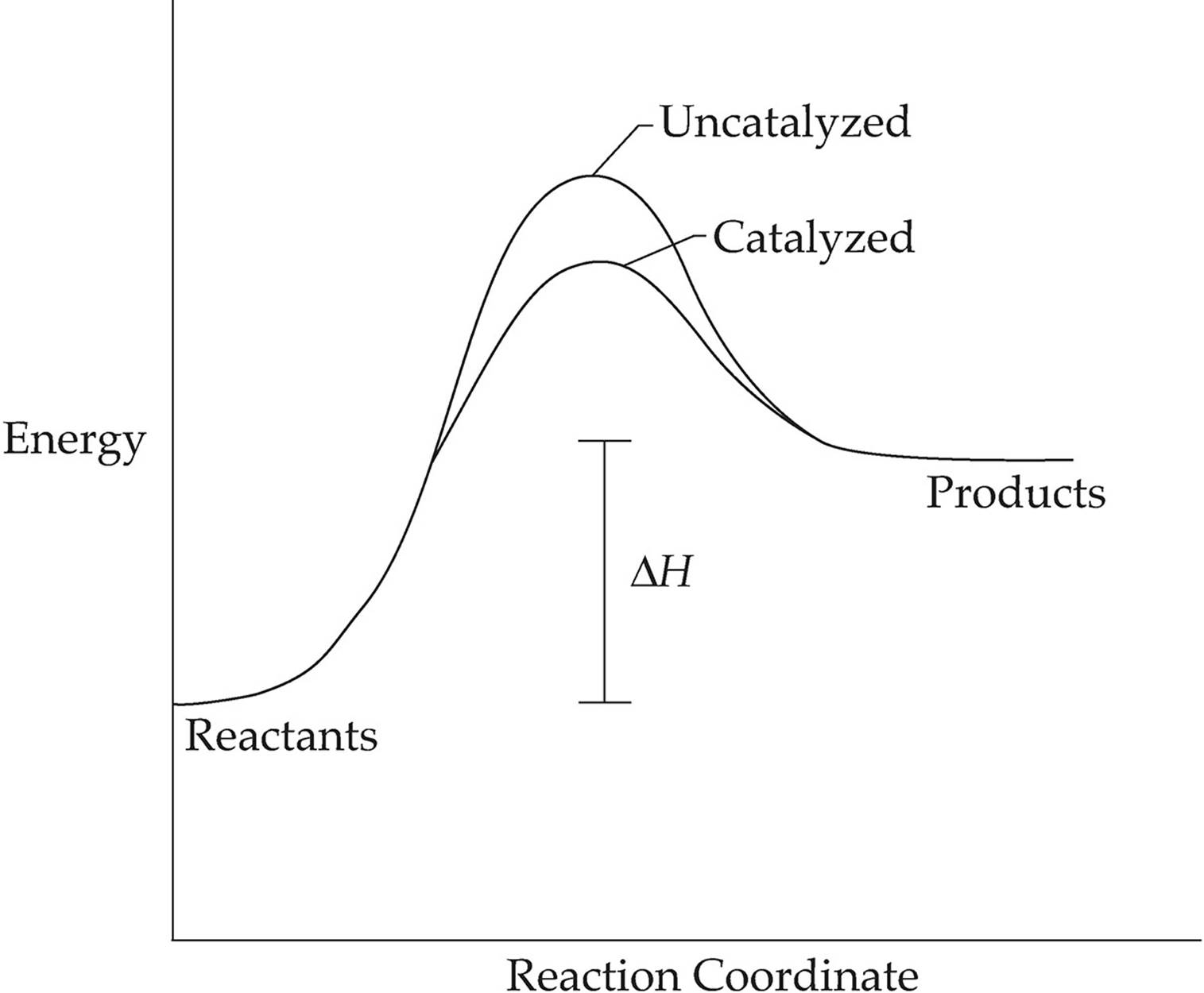
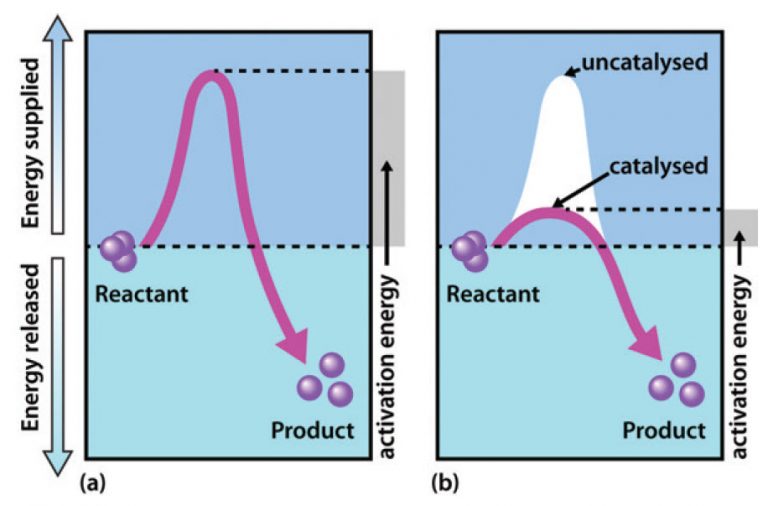
A catalyst speeds up a reaction by providing the reactants with an

savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts
A Catalyst Like Iodine Can Be Used To Provide An Alternate Pathway For The Reaction With A Much Lower Activation Energy Of Approximately 118 Kj/Mol (Figures 17.15 “Catalyzed.
The Catalyst Does Not Affect The Energy Of The Reactants Or Products (And Thus Does Not Affect Δ E ).
Web Reaction Diagrams For Catalyzed Reactions.
A Catalyzed Mechanism Must Involve At Least Two Steps, One Where The Catalyst Interacts With A Reactant To Form An.
Related Post: