How To Draw Bohr Model
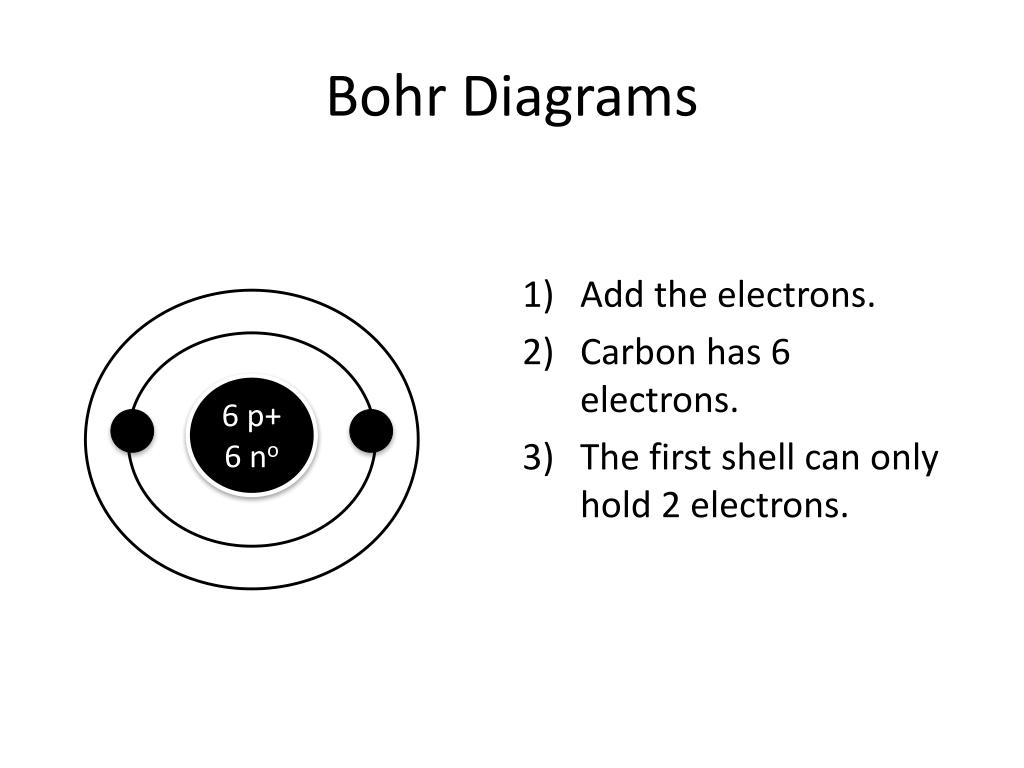
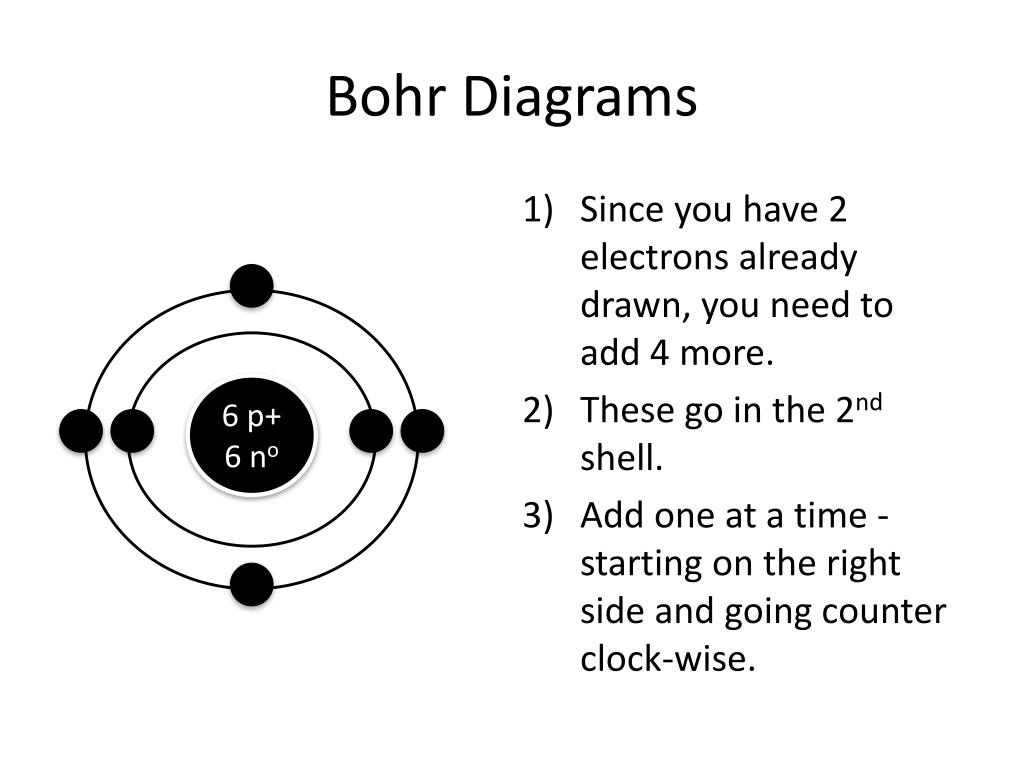
How To Draw Bohr Model - These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. The maximum number of electrons that can fill each shell is: Bohr's model calculated the following energies for an electron in the shell, n : Draw the next shell if you have more electrons to add 6. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. #2 draw nucleus of cobalt atom. Web how to draw bohr model of an atom? Web bohr model energy levels. Let’s break down each step in detail. Write the number of protons and neutrons in the nucleus 3. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Draw the nucleus of an atom. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. E ( n). Deriving the bohr radius of the atom. This packet includes a video explaining the bohr model and how to create and use bohr model diagrams, as well as practice problems. Web the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Web. #5 draw 3 rd electron shell. Web in the bohr model, there are a few rules that will help you draw accurate diagrams. Web key points bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. #1 write protons, neutrons, and electrons of cobalt atom. Electrons can jump from. Draw the electrons in their respective orbitals. Learn how to draw a bohr model with help from an artist in this free. Electrons must occupy the lowest available shell, closest to the nucleus. The number of shells that you draw will depend on the number of electrons in the atom. Web how to draw bohr model of an atom? Sodium (na) 2, 8, 1: Draw the first electron shell and put the electrons as a dot in it. Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. Draw the electrons in their respective orbitals. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. #2 draw nucleus of cobalt atom. #1 write protons, neutrons, and electrons of copper atom #2 draw nucleus of copper atom #3 draw 1 st electron shell #4 draw 2 nd electron shell #5 draw 3 rd electron shell #6 draw 4 th electron shell. Web steps to draw bohr model of boron. The protons and neutrons are placed into. Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. Web this video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with examples. Deriving the bohr radius of the atom. You will also learn how to write bohr model electron configuration as. Draw the electrons in their respective orbitals. To evaluate the number of valence electrons using a bohr model. Discuss how the bohr model can be used to explain atomic spectra. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. • the atomic number of boron is 5. Bohr described the hydrogen atom in terms. #2 draw nucleus of cobalt atom. Web in the bohr model of the atom, electrons travel in defined circular orbits around the nucleus. Web key points bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web this video will show you how to draw bohr model of atom. #5 draw 3 rd electron shell. Sodium (na) 2, 8, 1: The boron family is located in the 13 th group of the periodic table: The orbits are labeled by an integer, the quantum number n. Web bohr model energy levels. E ( n) = − 1 n 2 ⋅ 13.6 ev The orbits are labeled by an integer, the quantum number n. This packet includes a video explaining the bohr model and how to create and use bohr model diagrams, as well as practice problems. The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: Web how to draw bohr model of an atom? The boron family is located in the 13 th group of the periodic table: Draw a circle to represent the nucleus of the atom. Electrons can jump from one orbit to another by emitting or absorbing energy. The bohr model and photon wavelength. #1 write protons, neutrons, and electrons of cobalt atom. Draw the second electron shell,. Draw the first electron shell and put the electrons as a dot in it. Web the bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms. Draw the first shell add the electrons, the first shell can only hold 2 5. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Learn how to draw a bohr model with help from an artist in this free.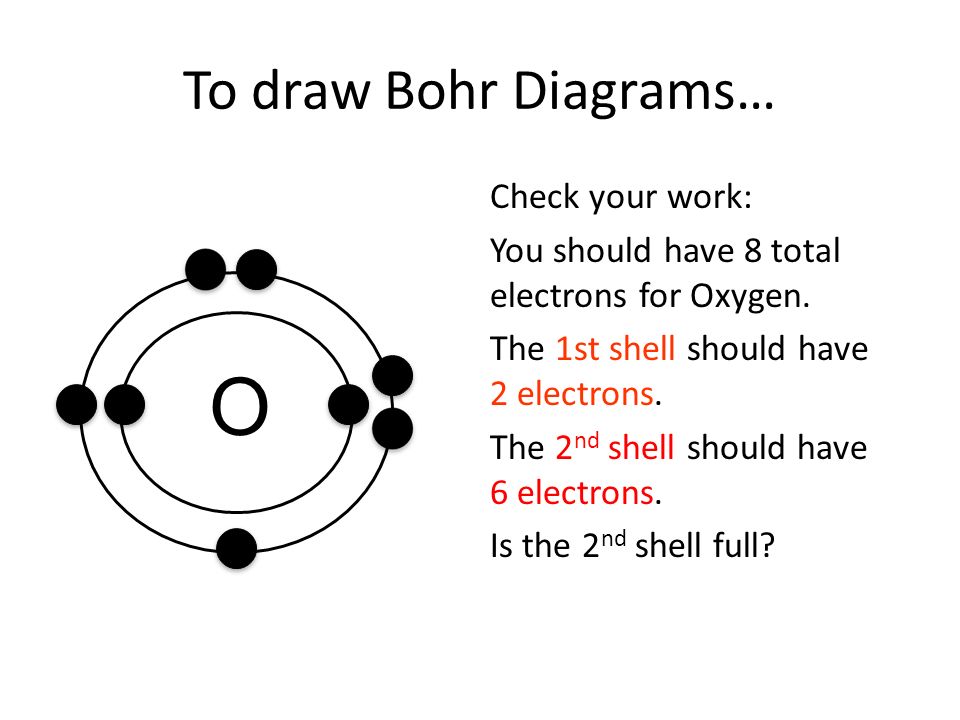
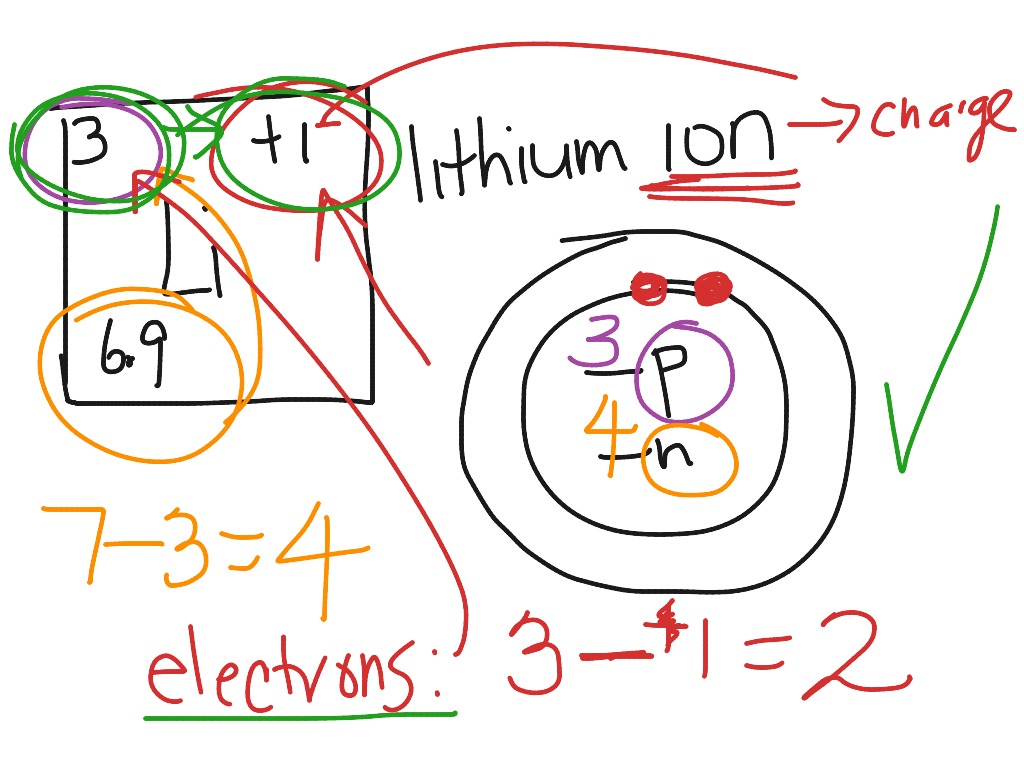
How To Draw A Bohr Model For An Ion Riset
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
Bohr Model Drawing Oxygen at GetDrawings Free download
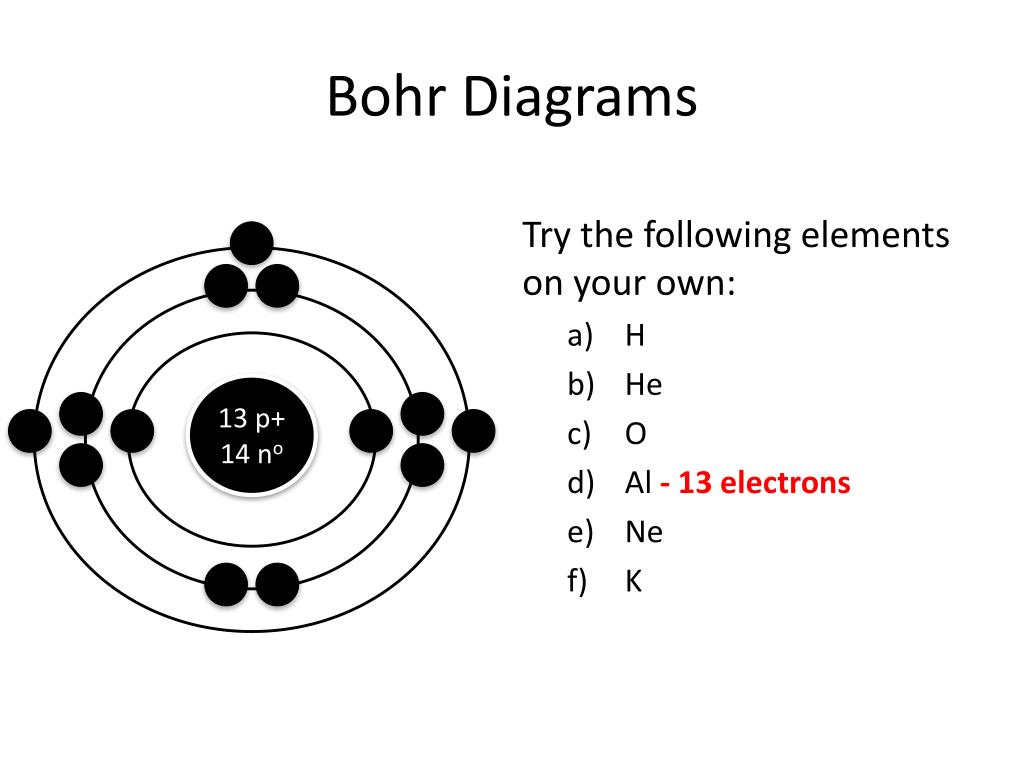
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
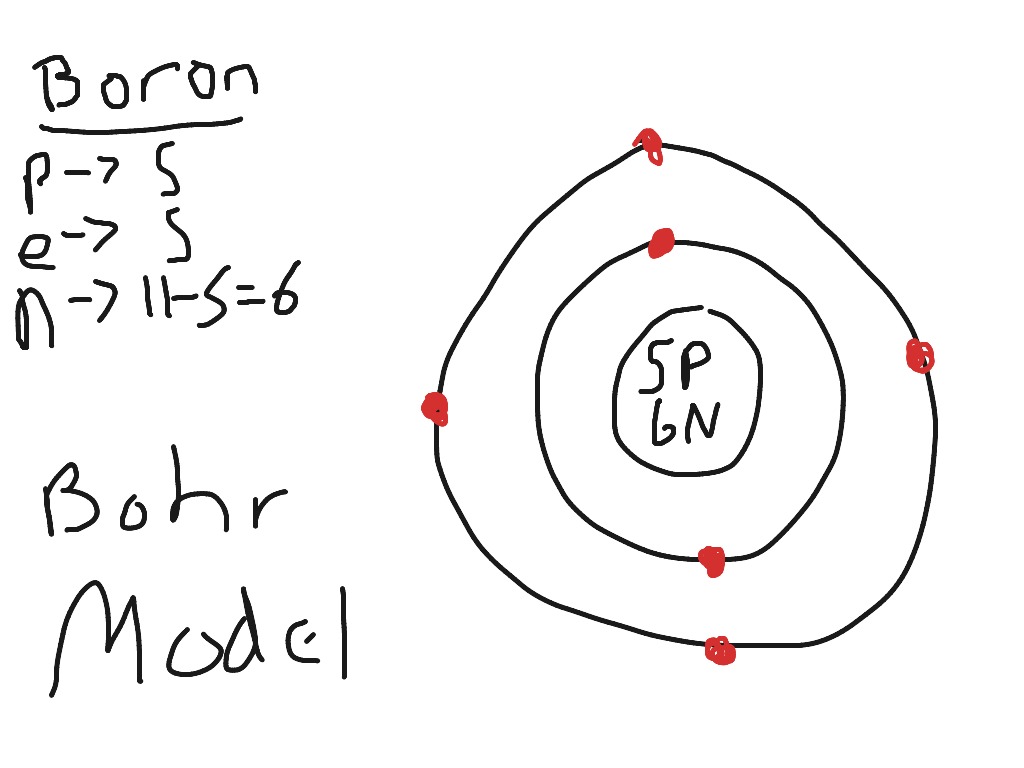
Boron Bohr Diagram
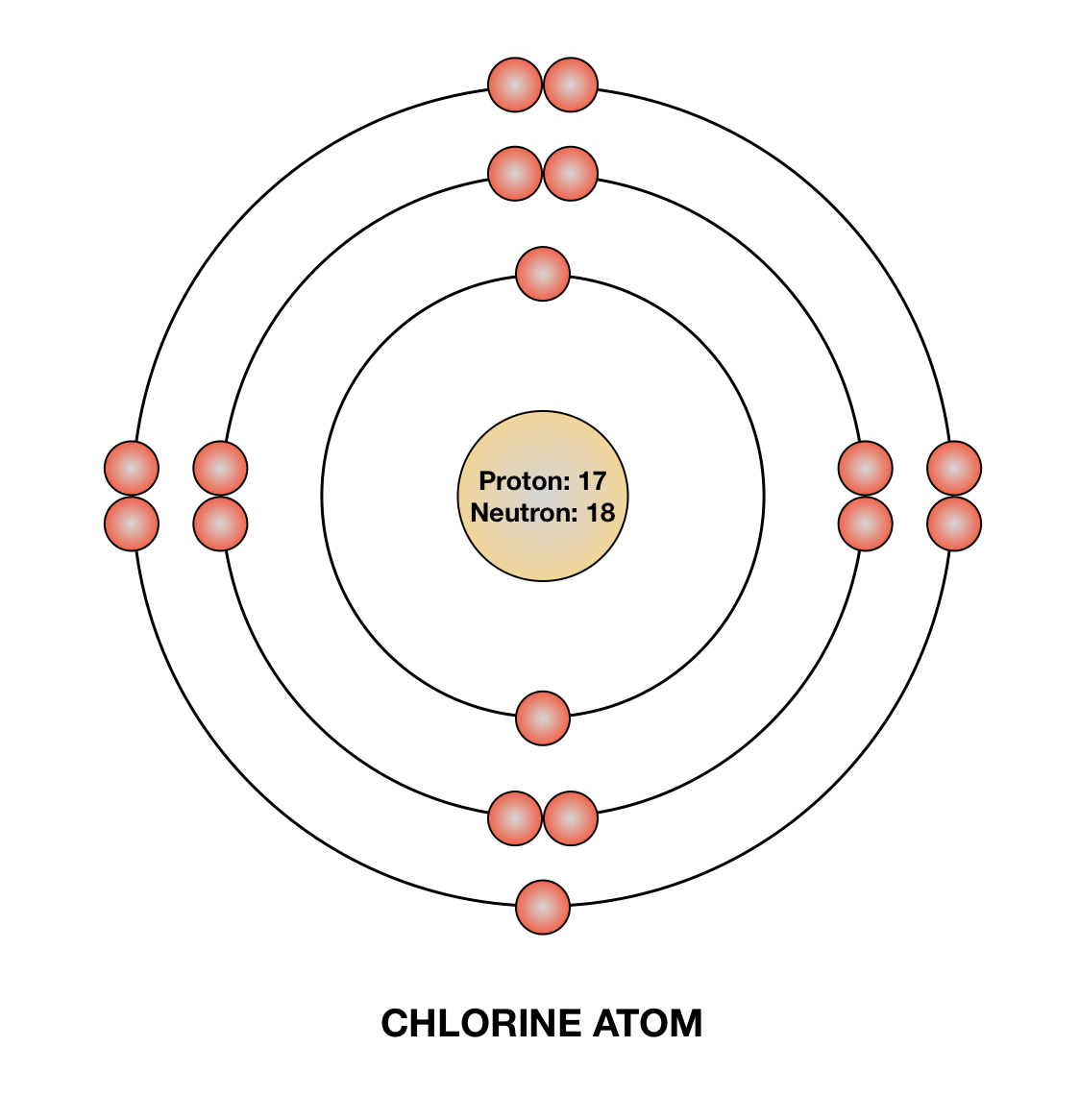
Draw a Bohr diagram of chlorine. Quizlet
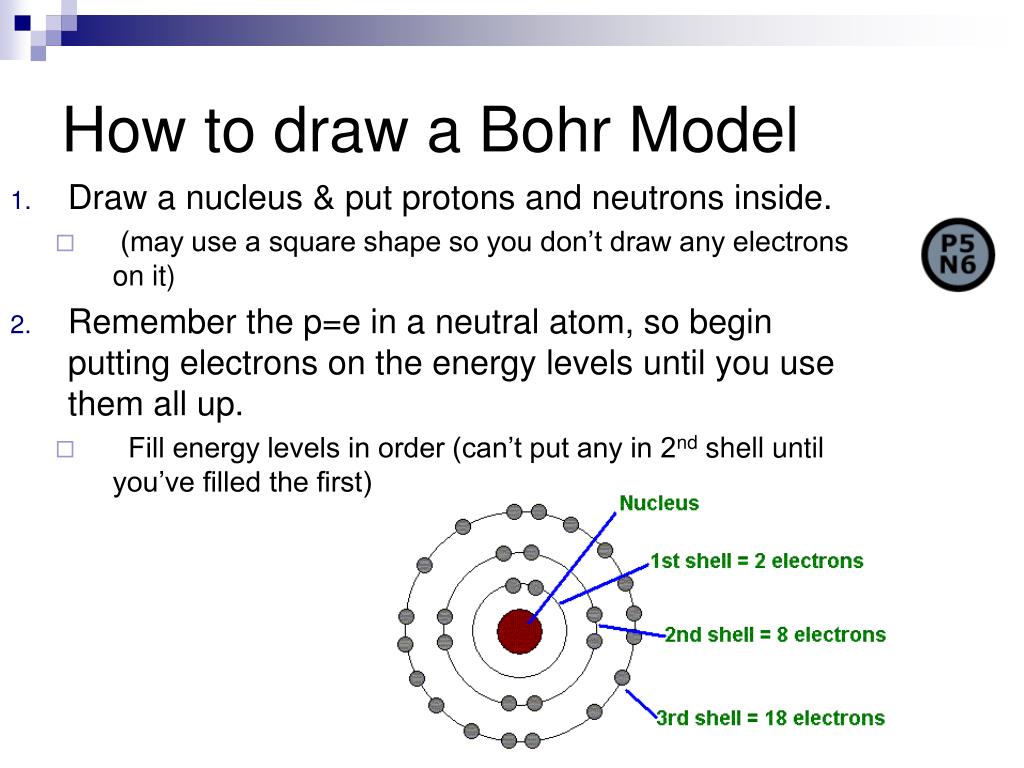
PPT Intro to Chemistry PowerPoint Presentation, free download ID

Carbon Bohr Model — Diagram, Steps to Draw Techiescientist

This video explains how to draw Lithium Bohr Model. It shows the number
• The Atomic Number Of Boron Is 5.
#4 Draw 2 Nd Electron Shell.
Web In The Bohr Model Of The Atom, Electrons Travel In Defined Circular Orbits Around The Nucleus.
Web The Bohr Model Shows The Atom As A Central Nucleus Containing Protons And Neutrons With The Electrons In Circular Orbitals At Specific Distances From The Nucleus (Figure \(\Pageindex{1}\)).
Related Post: