How To Draw Carbon Atom
How To Draw Carbon Atom - Each straight line segment represents a bond, the ends and intersections of the lines are carbon atoms, and the correct number of hydrogens is calculated from the tetravalency of carbon. This video discusses the resonance structure of this polyatomic ion as well as the. In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web if you want (or need) to draw a model of an atom, we'll show you how! And oxygen is in group six, so six valence electrons for oxygen. Determine the total number of electrons in the carbon and oxygen valence shells. There are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Let’s draw and understand this lewis dot structure step by step. 98k views 2 years ago. Carbon has 2 electrons in its first shell and 4 in its second shell. In the first step, we will draw the nucleus of the carbon atom. Atom labels for all other elements are shown. Calculation of valence electrons in co2 for carbon: There are enough hydrogen atoms attached to each carbon to make the total number of bonds on. Web how to draw human cell step by step. Carbon is a member of the iva group and has four electrons in its valence shell. Calculation of valence electrons in co2 for carbon: Lone pair electrons are usually omitted. For this, we will first have to calculate the number of protons and neutrons present in this atom. Determine the total number of electrons in the carbon and oxygen valence shells. 98k views 2 years ago. In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web steps to draw the bohr model of carbon atom 1. So, let’s calculate this first. Web 0:00 / 1:37 lewis dot structure of co2 (carbon dioxide) kentchemistry.com 25.1k subscribers 225k views 12 years ago every video i quickly take you through how. In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web if you want (or need) to draw a model of an atom, we'll show. 98k views 2 years ago. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Bohr diagrams show electrons orbiting the nucleus. Take a pen and paper with you and try to draw this lewis structure along with me. Web here's hydrogen, in group one, so one valence electron. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. We have one atom of carbon, so that's four valence electrons. Carbon. Web here's hydrogen, in group one, so one valence electron. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Atom labels for all other elements are shown. (valence electrons are the number of electrons present in the outermost shell of an atom).. Carbon is a member of the iva group and has four electrons in its valence shell. So let's go back down and let's calculate the total number of valence electrons that we need to represent in our dot structure. Hydrogens that are attached to elements other than carbon are shown. Because hydrogen only needs two. However, conventionally, we draw the. 98k views 2 years ago. In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web how to draw human cell step by step. So, let’s calculate this first. (valence electrons are the number of electrons present in the outermost shell of an atom). Calculation of valence electrons in co2 for carbon: However, conventionally, we draw the dots. We have one atom of carbon, so that's four valence electrons. Take a pen and paper with you and try to draw this lewis structure along with me. Web shorthand (line) formulas omit the symbols for carbon and hydrogen entirely. In the bohr model, electrons are pictured as traveling in circles at different shells,. Video showing how to draw a carbon atom. We have one atom of carbon, so that's four valence electrons. Questions tips & thanks want to join the conversation? Web if you want (or need) to draw a model of an atom, we'll show you how! Calculation of valence electrons in co2 for carbon: Web shorthand (line) formulas omit the symbols for carbon and hydrogen entirely. Carbon is a member of the iva group and has four electrons in its valence shell. So let's go back down and let's calculate the total number of valence electrons that we need to represent in our dot structure. Each straight line segment represents a bond, the ends and intersections of the lines are carbon atoms, and the correct number of hydrogens is calculated from the tetravalency of carbon. Web 0:00 / 14:06 how to draw atoms (bohr model) highschoolscience101 36.1k subscribers 24k views 2 years ago chemistry in this lesson i present an overview of: Web we will use this information to draw the bohr model of the carbon atom. Web a carbon atom is present wherever a line intersects another line. There are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. (valence electrons are the number of electrons present in the outermost shell of an atom). However, conventionally, we draw the dots.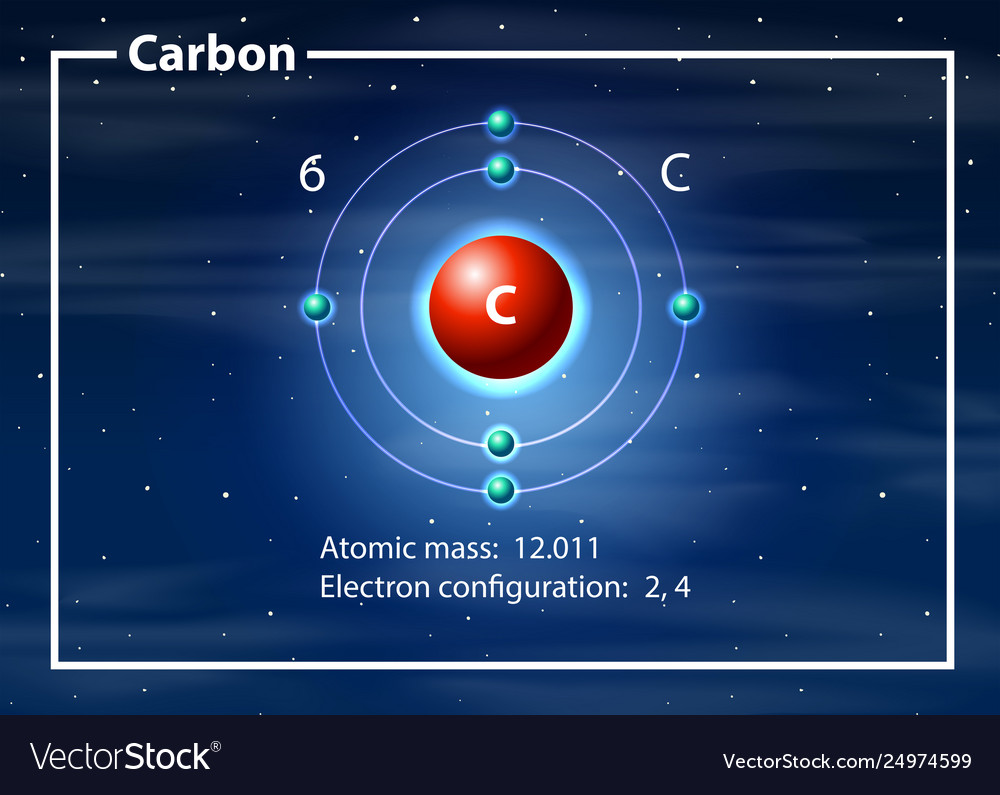
Carbon atom diagram concept Royalty Free Vector Image

Carbon atomic structure (437243) Illustrations Design Bundles
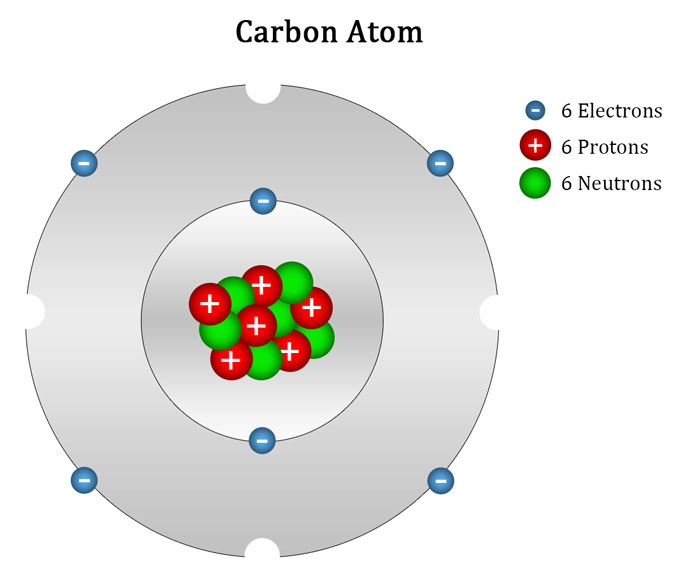
Carbon Atom Ascension Glossary

Basic Chemistry Tutorial 2, Drawing Atoms sciencemusicvideos
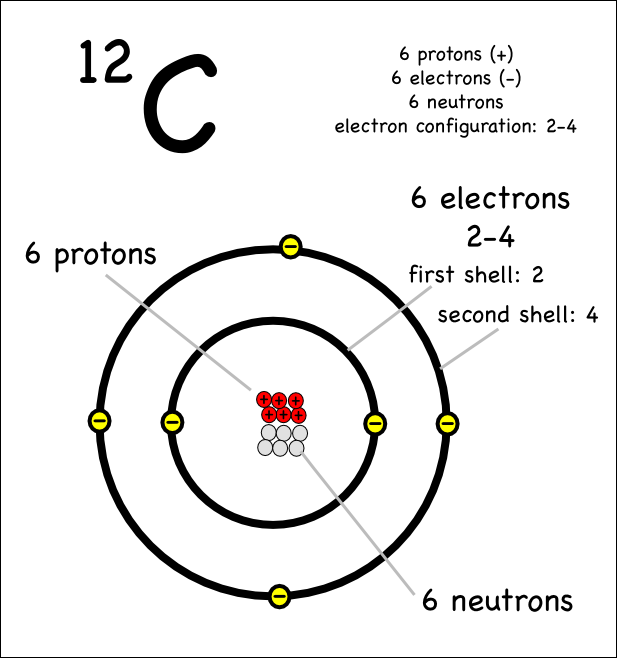
Drawing Atoms Montessori Muddle
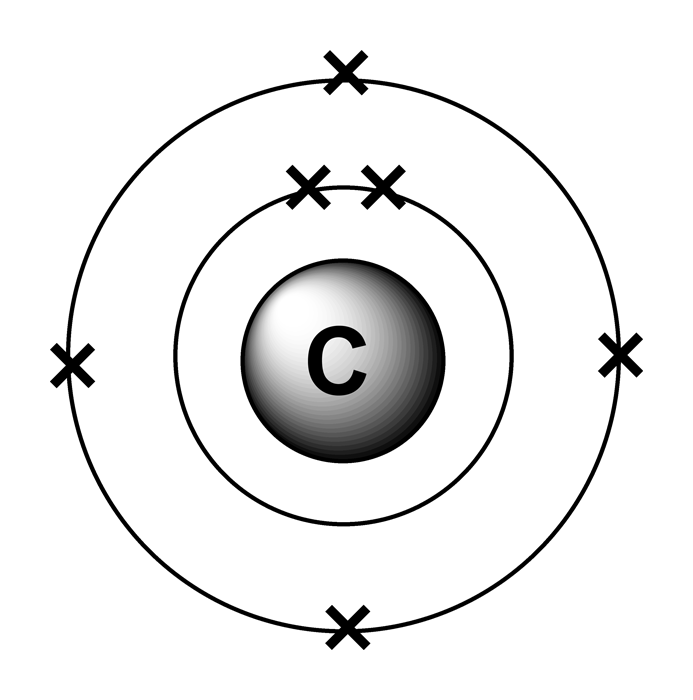
Electron configurations

How To Draw Carbon at How To Draw
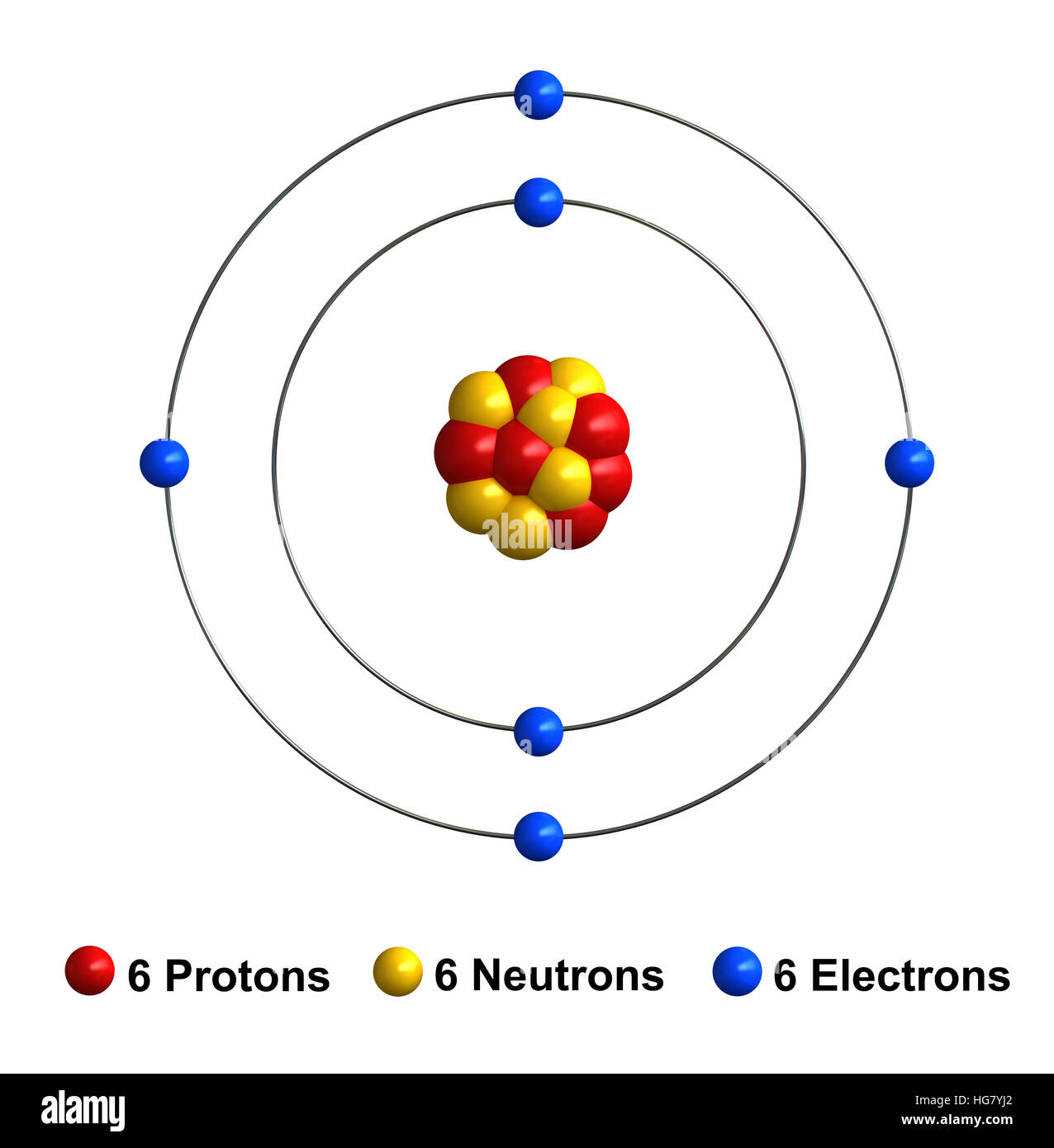
Carbon Atomic Structure High Resolution Stock Photography and Images

Draw and label the structure of an atom of carbon Brainly.in
![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/small/65382287-b5ac-4ea6-8a57-1f5a107b172c/a-carbon-atom---teachoo.jpg)
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
Web Here's Hydrogen, In Group One, So One Valence Electron.
And Oxygen Is In Group Six, So Six Valence Electrons For Oxygen.
In The First Step, We Will Draw The Nucleus Of The Carbon Atom.
Connect The Atoms To Each Other With Single Bonds To Form A “Skeleton Structure.” Be Sure That You Follow Rule 1.
Related Post: