How To Draw Hydrogen Atom
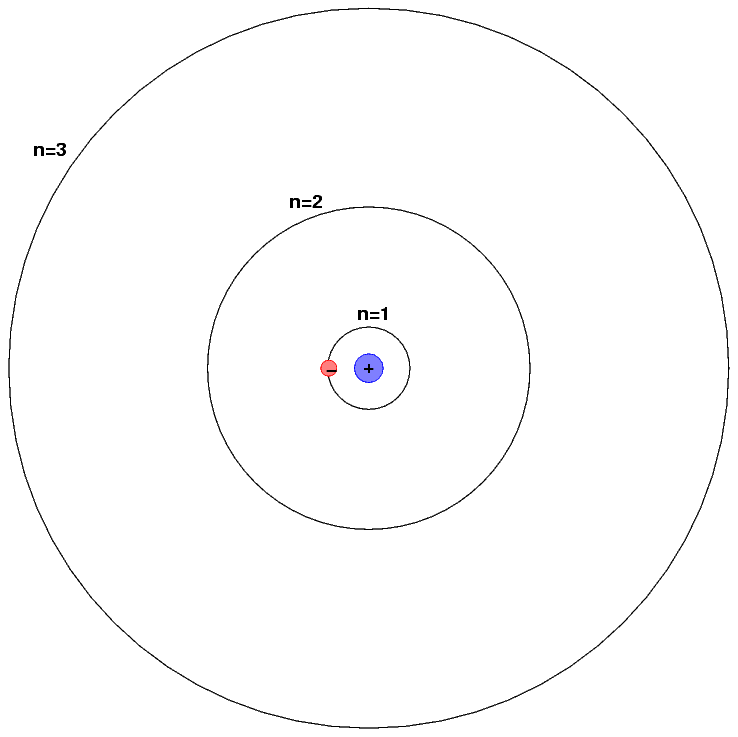
How To Draw Hydrogen Atom - In order to draw the lewis structure of h2, first of all you have to find the total number of valence electrons present in the h2 molecule. Web (hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. Web using conventional bond notation, you could draw it as, for example: Make sure the shape is correct. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. In general we try to get the octet rule for any atom except for hydrogen. E ( n) = − 1 n 2 ⋅ 13.6 ev Add enough electrons (dots) to the outer atoms to. As the most electronegative element, fluorine also cannot be a central atom. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. This video will show you how to draw atoms of the first 20 elements by reading element information from nuclear notation and from. The most common element in. So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. 5.4k views 7 years ago. Calculate the total number of valence electrons. Either draw a line diagram or show every single atom and bond. Each oxygen atom has two bonds. Add enough electrons (dots) to the outer atoms to. Web and to draw the atoms you fill up the inner shells first then move on to the outer shells. Web the hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. Each hydrogen atom has one bond. It results in the emission of. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h. Each oxygen atom has two bonds. Web the hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. In general we try to get the octet rule for any atom except for hydrogen. Figure out. Each carbon atom has four bonds. Web using conventional bond notation, you could draw it as, for example: Add enough electrons (dots) to the outer atoms to. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Calculate the total number of valence electrons. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. For the h2 structure use the periodic table to find the total number of valence electrons f.more.more.. This is shown in the two models below. Make sure the shape is correct. Web for the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 å (52.9 pm), which is exactly the radius calculated by bohr for the n = 1 orbit. Each nitrogen atom has three bonds. Here, the given molecule is h2. Web and to draw the atoms you fill up the inner shells first then move on to the outer shells. The radius of the first bohr orbit is called the bohr radius of hydrogen, denoted as a0. Its value is obtained by setting n = 1 in equation 6.5.6: In general we try to get the octet rule for any. In general we try to get the octet rule for any atom except for hydrogen. Web for the hydrogen atom, the peak in the radial probability plot occurs at r = 0.529 å (52.9 pm), which is exactly the radius calculated by bohr for the n = 1 orbit. Hydrogen, you just need to get to two in that outer. Each carbon atom has four bonds. Web the hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. Web in this video we'll look at the. Calculate the total number of valence electrons. Therefore, it is important to further study the grating formation mechanism of fiber gratings for the rapid. Distinguish between the bohr and schrödinger models of the atom Web and to draw the atoms you fill up the inner shells first then move on to the outer shells. As the most electronegative element, fluorine also cannot be a central atom. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web steps to draw the lewis structure of h2 step #1: The radius of the first bohr orbit is called the bohr radius of hydrogen, denoted as a0. Its value is obtained by setting n = 1 in equation 6.5.6: So, if i wrote just the element symbol and its atomic mass on the board that students should be able to figure out the number of particles. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. This is shown in the two models below. We’ll use a bohr diagram to visually represent where the electrons are around the nucleus of the h. A0 = 4πϵ0 ℏ2 mee2 = 5.29 × 10 − 11m = 0.529 å. The collective states of all electrons in an atom having the same principal quantum number (visualized as an orbit in which the electrons move). It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms.
Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy
Bohr Model of the Hydrogen Atom Chemistry Steps
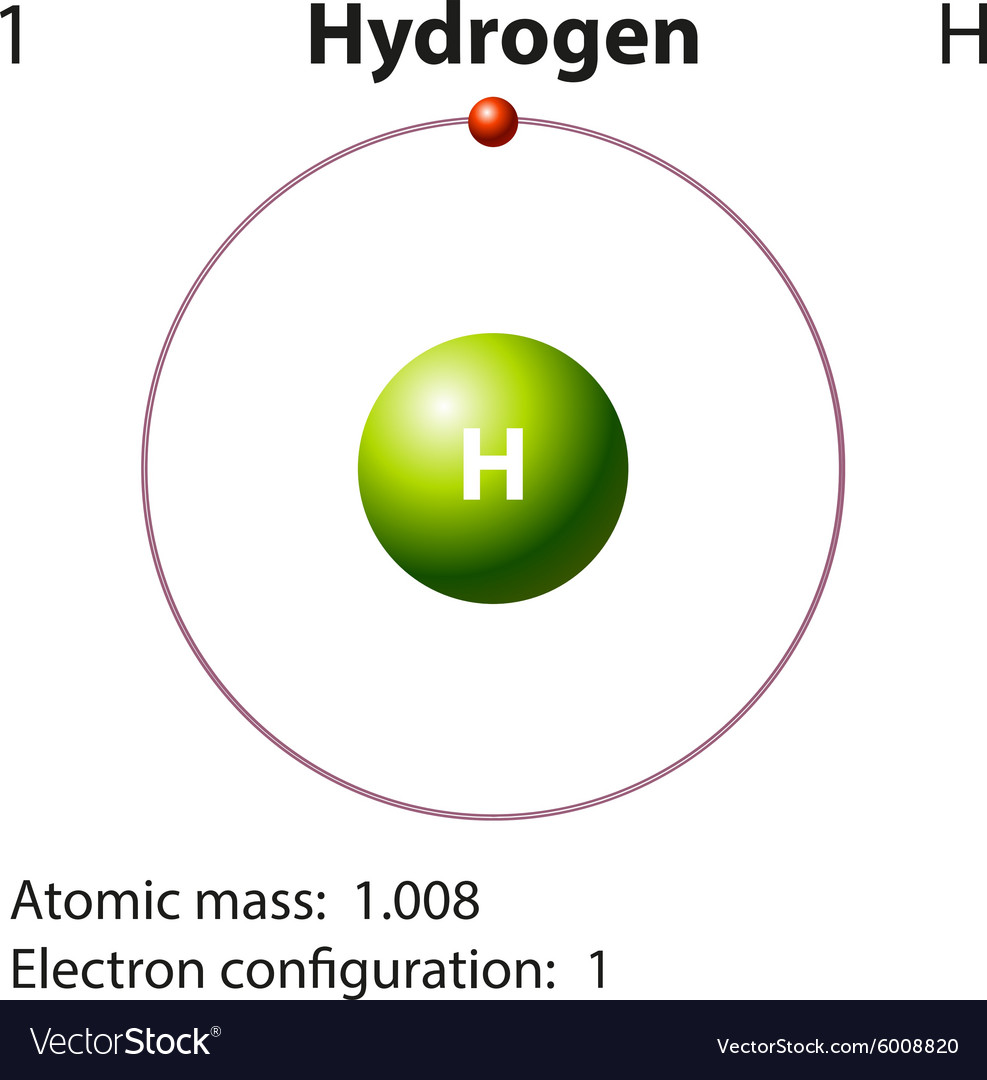
Diagram representation element hydrogen Royalty Free Vector

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps

Hydrogen Definition, Structure, Properties & Uses Embibe
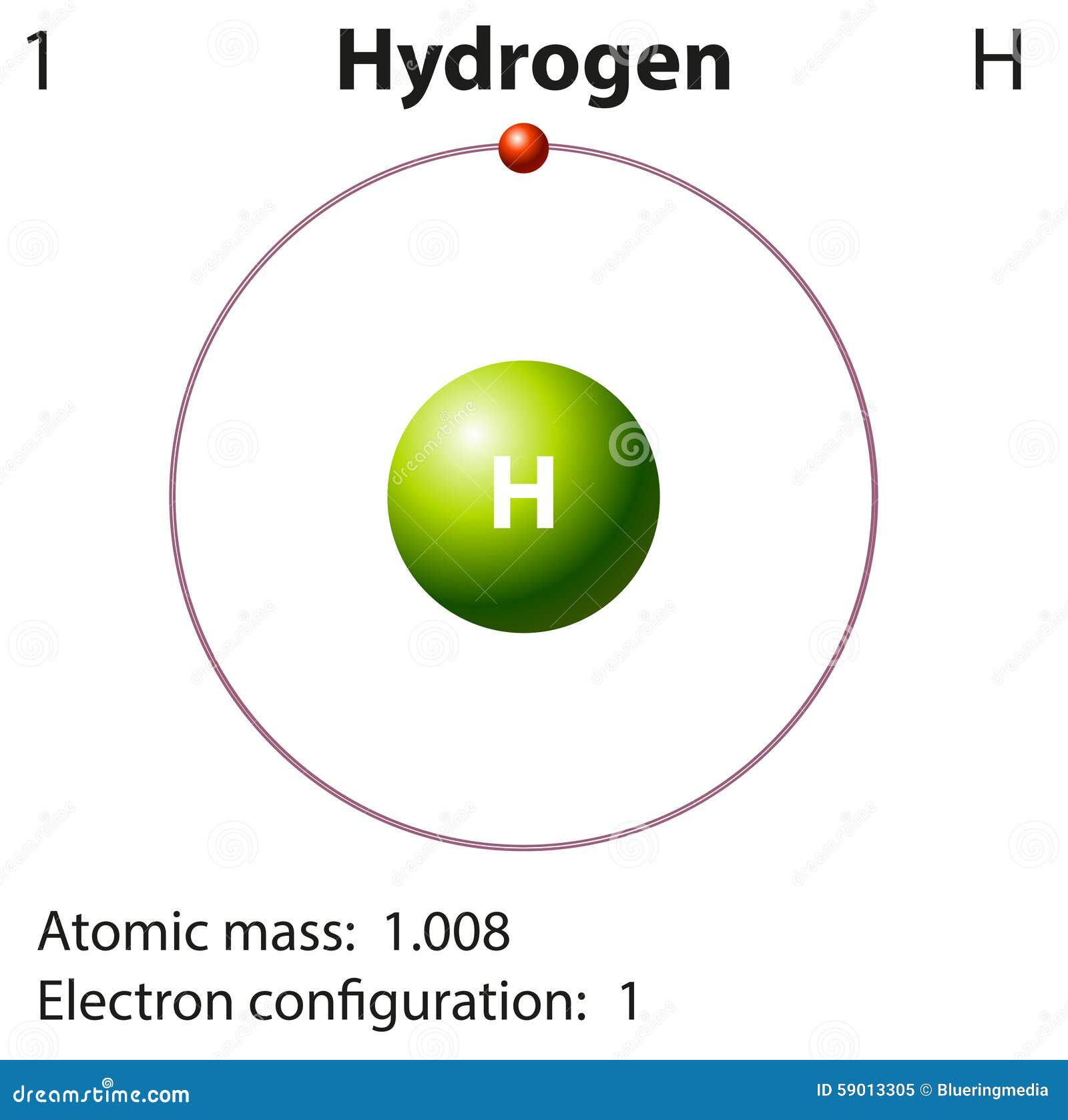
Diagram Representation Of The Element Hydrogen Stock Vector Image
GJ Blogs the atomic structure of hydrogen

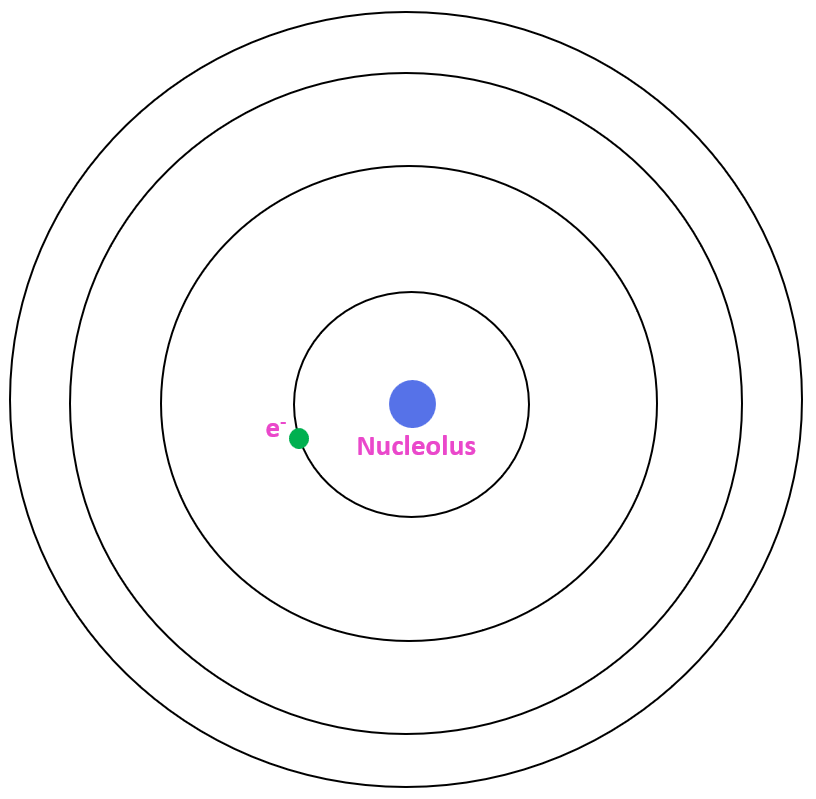
Hydrogen Atom Diagram

Hydrogen atom on white background Royalty Free Vector Image

Describe Bohr’s model of the hydrogen atom. bitWise Academy
Web In This Video We'll Look At The Atomic Structure And Bohr Model For The Hydrogen Atom (H).
Each Oxygen Atom Has Two Bonds.
Distribute The Remaining Electrons As Lone Pairs On The Terminal Atoms (Except Hydrogen) To Complete Their Valence Shells With An Octet Of.
Web Describe The Hydrogen Atom In Terms Of Wave Function, Probability Density, Total Energy, And Orbital Angular Momentum;
Related Post: