How To Draw Hydrogen Bonding
How To Draw Hydrogen Bonding - The examples that follow are representative of several types of biopolymers. Hydrogen bonds are shown with dotted lines. As a result, hydrogen bonds are a unique type of intermolecular attractive force that only occurs when hydrogen atoms are bound to a. • ( 19 votes) defranco.sal 8 years ago Learn how to study the hydrogen bond in chemical systems using avogadro. Explore hydrogen bonds forming between polar molecules, such as water. Web consider two water molecules coming close together. Web the commonest way to draw structural formulae. Web how to draw a hidrogen bond or a short contact in chembio3d? Because they are very strong, water is a liquid over a much wider temperature range than we would expect otherwise. Web consider two water molecules coming close together. Because they are very strong, water is a liquid over a much wider temperature range than we would expect otherwise. Hydrogen bond is an attraction force that formed between 2. Explore hydrogen bonds forming between polar molecules, such as water. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly negative atom, usually oxygen or nitrogen. Water molecules are also attracted to other polar molecules and to ions. It exists where one of the most electronegative elements. Sometimes the bonding is intramolecular, or between atoms of a molecule, rather than between atoms of separate molecules (intermolecular).. Try changing the temperature of the model. The examples that follow are representative of several types of biopolymers. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. Usually, hydrogen bonds occur between hydrogen and fluorine, oxygen, or nitrogen. • the more electronegative element has as least one lone pair. • ( 19 votes) defranco.sal 8 years ago In a molecule, when a hydrogen atom is linked to a highly electronegative atom, it attracts the shared pair of electrons more, and so this end of the molecule becomes slightly negative while the other end becomes slightly positive. Hydrogen bond is an attraction force that formed between 2. Learn how to. Web hydrogen bonding between different parts of the same chain (intramolecular bonding; Web criteria for hydrogen bonding • the hydrogen atom is attached to an element with a high electronegativity (n, o, or f). Learn how to study the hydrogen bond in chemical systems using avogadro. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly. • the more electronegative element has as least one lone pair. Web hydrogen bonds drawing and explanation. Explore hydrogen bonds forming between polar molecules, such as water. Usually, hydrogen bonds occur between hydrogen and fluorine, oxygen, or nitrogen. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Sometimes the bonding is intramolecular, or between atoms of a molecule, rather than between atoms of separate molecules (intermolecular). Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web consider two water molecules coming close together. Show partial charges and run the model. In a molecule, when a. Water molecules are also attracted to other polar molecules and to ions. Web hydrogen bonding occurs between molecules in which a hydrogen atom is attached to a strongly electronegative element: Show partial charges and run the model. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. In a molecule, when a hydrogen atom is. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. In a molecule, when a hydrogen atom is linked to a highly electronegative atom, it attracts the shared pair of electrons more, and so this end of the molecule becomes slightly negative while the other end becomes slightly positive.. Water molecules are also attracted to other polar molecules and to ions. Sometimes the bonding is intramolecular, or between atoms of a molecule, rather than between atoms of separate molecules (intermolecular). Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web draw hydrogen bonds between one water molecule. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. This video shows three examples of drawing for the formation of hydrogen bond. Web hydrogen bonds are formed between a slightly positive hydrogen atom and a slightly negative atom, usually oxygen or nitrogen. Learn how to study the hydrogen bond in chemical systems using avogadro. I need to specify the conformer/stereoisomer by adding intramolecular hydrogen bonds. It exists where one of the most electronegative elements. How does the pattern of hydrogen bonding explain the lattice. Web hydrogen bonding between two water molecules is represented by the dashed lines. Show partial charges and run the model. Web hydrogen bonding between different parts of the same chain (intramolecular bonding; Web consider two water molecules coming close together. Explore hydrogen bonds forming between polar molecules, such as water. Web criteria for hydrogen bonding • the hydrogen atom is attached to an element with a high electronegativity (n, o, or f). Web how to draw a hidrogen bond or a short contact in chembio3d? Web the commonest way to draw structural formulae. Usually, hydrogen bonds occur between hydrogen and fluorine, oxygen, or nitrogen.
Hydrogen Bonding Definition, Example, Types, Question Embibe

Hydrogen Bonds — Overview & Examples Expii

Primary and Secondary Bonds Owlcation
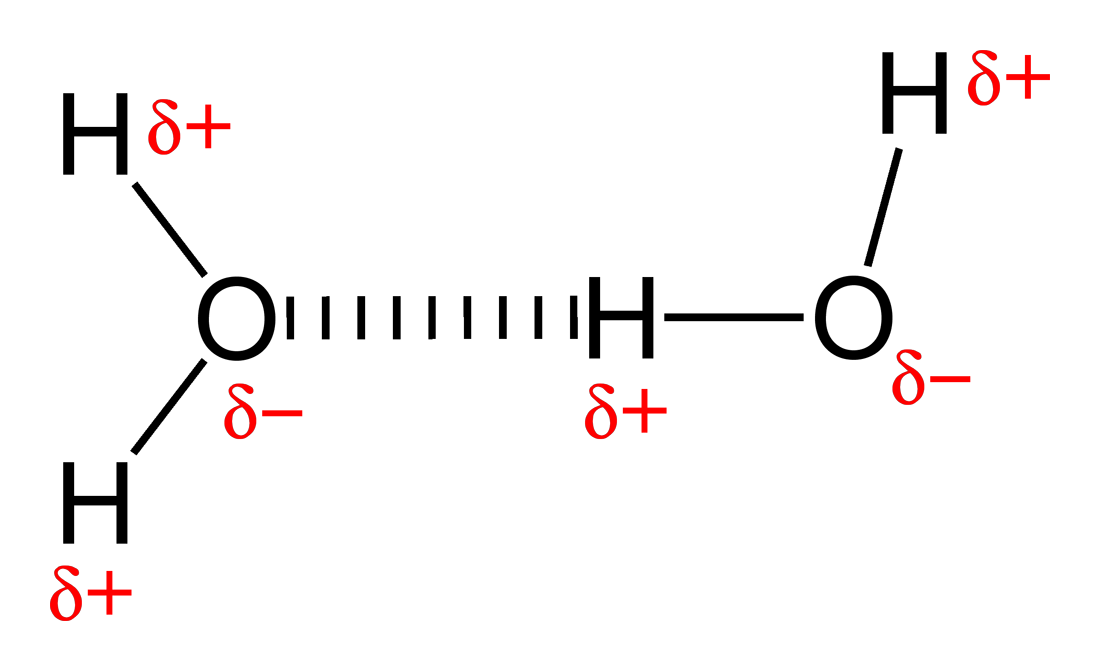
The Curious Wavefunction A bond by any other name... How the simple definition of a hydrogen
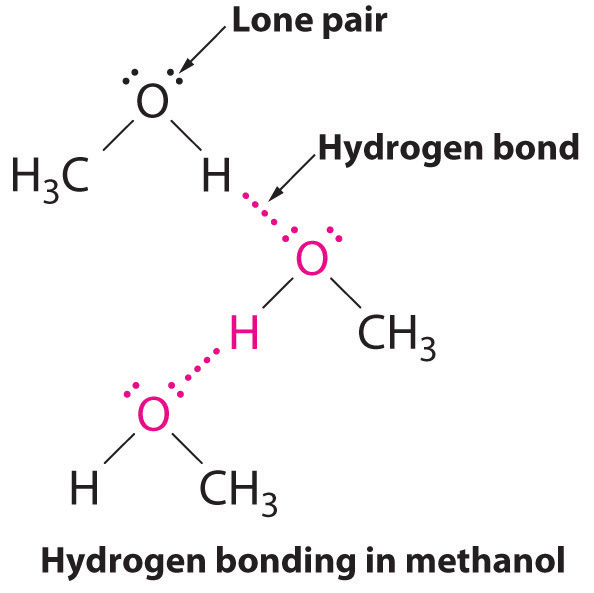
Intermolecular Forces

Water has both a hydrogen bond and a polar covalent bond. Hydrogen bond, Covalent bonding

savvychemist Intermolecular Forces (3) Hydrogen Bonding

Diagram Of Water Molecules Hydrogen Bonding
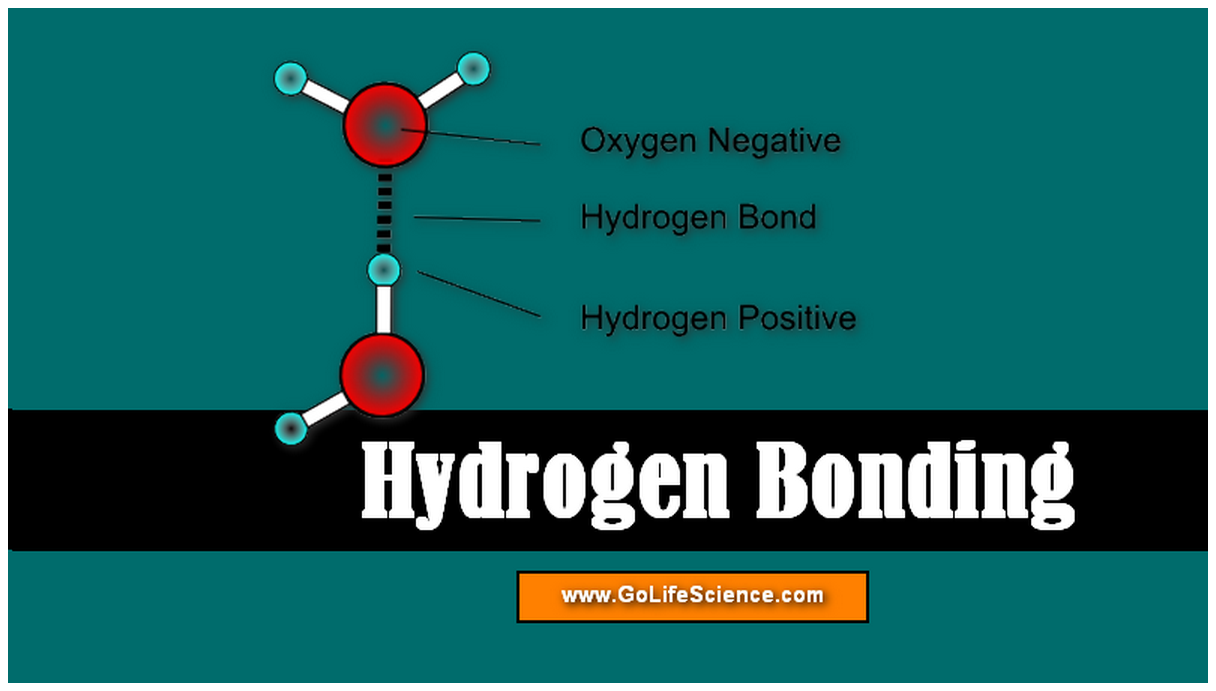
Hydrogen Bonding What is Hydrogen bonding and its types?
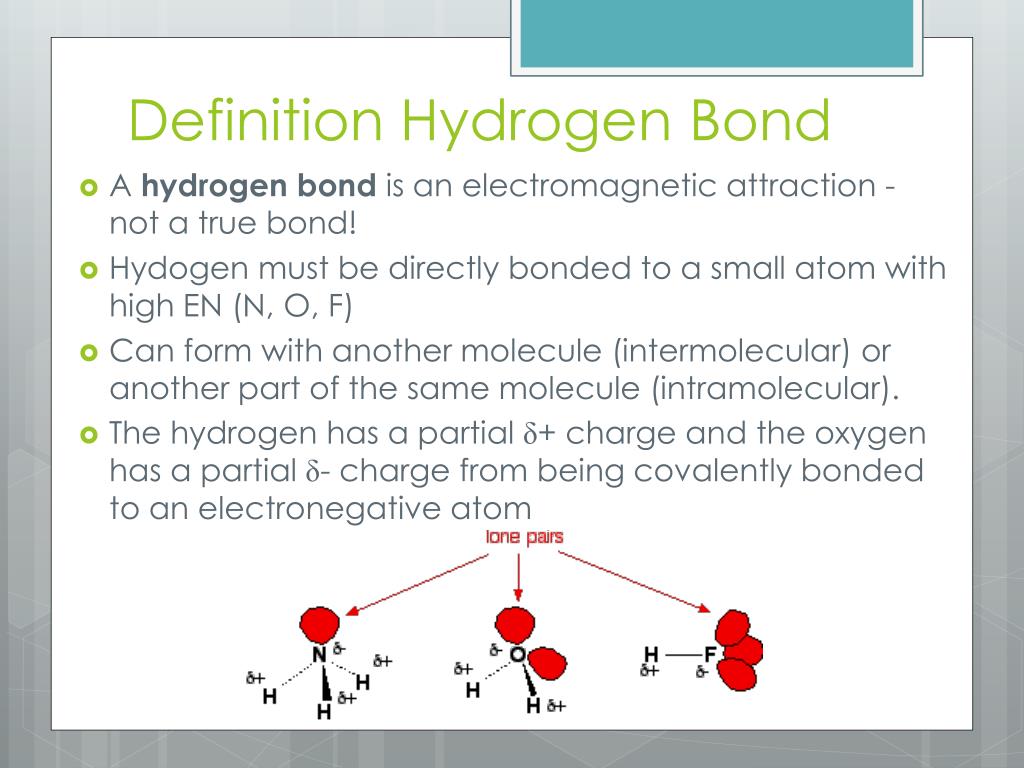
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Web Hydrogen Bonding Occurs Between Molecules In Which A Hydrogen Atom Is Attached To A Strongly Electronegative Element:
In A Molecule, When A Hydrogen Atom Is Linked To A Highly Electronegative Atom, It Attracts The Shared Pair Of Electrons More, And So This End Of The Molecule Becomes Slightly Negative While The Other End Becomes Slightly Positive.
Web The Partial Negative Charge On The O Of One Molecule Can Form A Hydrogen Bond With The Partial Positive Charge On The Hydrogens Of Other Molecules.
Add Enough Electrons (Dots) To The Outer Atoms To.
Related Post: