Surface Tension Drawing
Surface Tension Drawing - The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids. The stronger the intermolecular interactions, the greater the surface tension. Web the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension has the dimension of force per unit length, or of energy per unit area. I have seen various symbols, such as t, s t, s and γ γ used for surface tension. It combines the concepts of cohesion and adhesion. Web surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. If the surface line element is a closed loop Surface tension can be defined as σ = fs / l (1) where σ = surface tension (n/m) fs = stretching force (n) l = unit length (m) Acrylic paints aren’t the only types that work. Acrylic paints aren’t the only types that work. Web surface tension has the units of force per length, and its action is confined to the free surface. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). If the surface line element. Web the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension can be defined as σ = fs / l (1) where σ = surface tension (n/m) fs = stretching force (n) l = unit length (m) It is also responsible for the beading up of water droplets on. Web surface tension is an important factor in the phenomenon of capillarity. Web explore surface tension and how it varies from one liquid to another. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Acrylic paints aren’t the only types that work. The dimensions are mt −2. There is a common misconception for the source of the surface tension. Surfactants like detergent), each solution exhibits differing surface tension properties. Liquids that have strong intermolecular forces, like the hydrogen bonding in water, exhibit the greatest surface tension. The stronger the intermolecular interactions, the greater the surface tension. The directed contracting force which attracts the molecules at the surface. A surface line element d‘ will feel a total force σd‘ owing to the local surface tension σ(x). Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Ui = γa, (8.2.1) (8.2.1) u i = γ a, where a a is the interface area, and γ γ is called the surface. Surfactants are molecules, such as soaps and detergents, that reduce the surface tension of polar liquids like water. Web surface tension has the units of force per length, and its action is confined to the free surface. Web • surface tension is a property of a liquid that allows them to resist external forces. Gamma = f / d units. Web the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. This creates surface tension, which allows for phenomena such as water droplets maintaining a round shape and insects walking on water. Web surface tension is the energy required to increase the surface area of a liquid by a given amount.. Web surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. A surface line element d‘ will feel a total force σd‘ owing to the local surface tension σ(x). Surfactants like detergent), each solution exhibits differing surface tension properties. Web at the surface of water, molecules are more densely. Web explore surface tension and how it varies from one liquid to another. Web surface tension is the energy required to increase the surface area of a liquid by a given amount. Web surface tension is an important factor in the phenomenon of capillarity. Table 1 gives the value of the surface tension for some typical materials. Since these intermolecular. Surface tension not only depends upon the forces of attraction between the particles within the given liquid but also on the forces of attraction of. If the surface line element is a closed loop Web surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. There is a common misconception. Web surface tension allows objects that are denser than water, such as the paper clip shown in b in the figure below, to nonetheless float on its surface. Acrylic paints aren’t the only types that work. Web surface tension is defined as the energy required to increase the surface area of a liquid, or the force required to increase the length of a liquid surface by a given amount. Consider for the sake of simplicity a perfectly flat interface. Web the force per unit length perpendicular to a line drawn in the surface of the liquid is the surface tension. The dimensions are mt −2. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Web the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Web • surface tension is a property of a liquid that allows them to resist external forces. The surface tension of a liquid results from an imbalance of intermolecular attractive forces, the cohesive forces between molecules: The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids. An overview of intermolecular forces in action as surface tension, viscosity, and capillary action. Try different colors to see which ones show up well. In the cups, mix two or three paint colors with water in. Surface tension is caused by a strong attraction between the molecules (cohesion) that cause them to link together and remain uniform, even when placed on differing surfaces (adhesion). Web the surface tension of a liquid is a measure of the elastic force in the liquid’s surface.
Schematic illustration of standard methods of surface tension
Surface Tension Stock Illustration Download Image Now iStock

Explain the surface tension phenomenon with examples.
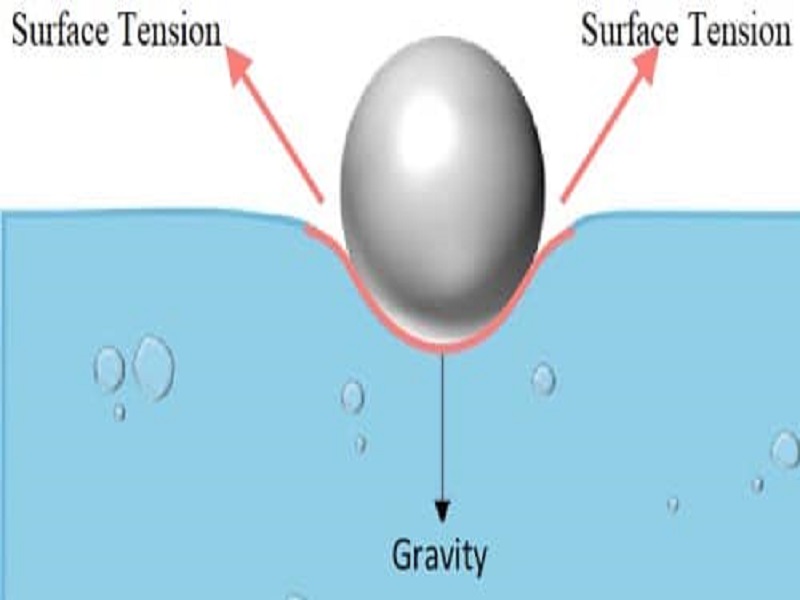
10 Surface Tension Examples in Daily Life StudiousGuy

Illustration of the modeling of the surface tension forces with the
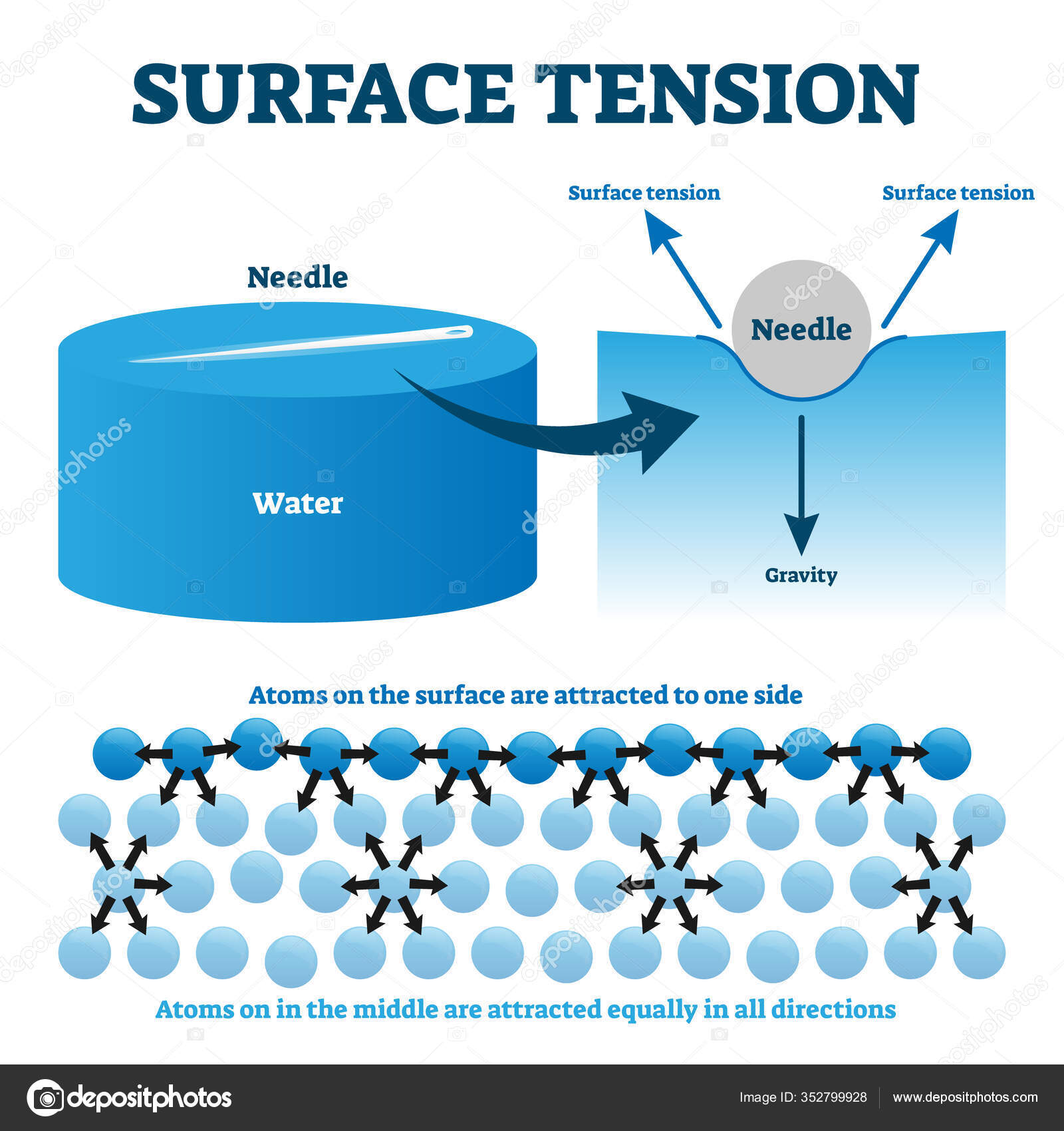
Surface tension explanation vector illustration diagram Stock Vector

Surface Tension Drawing & Painting on Aluminium Jackson's Art Blog
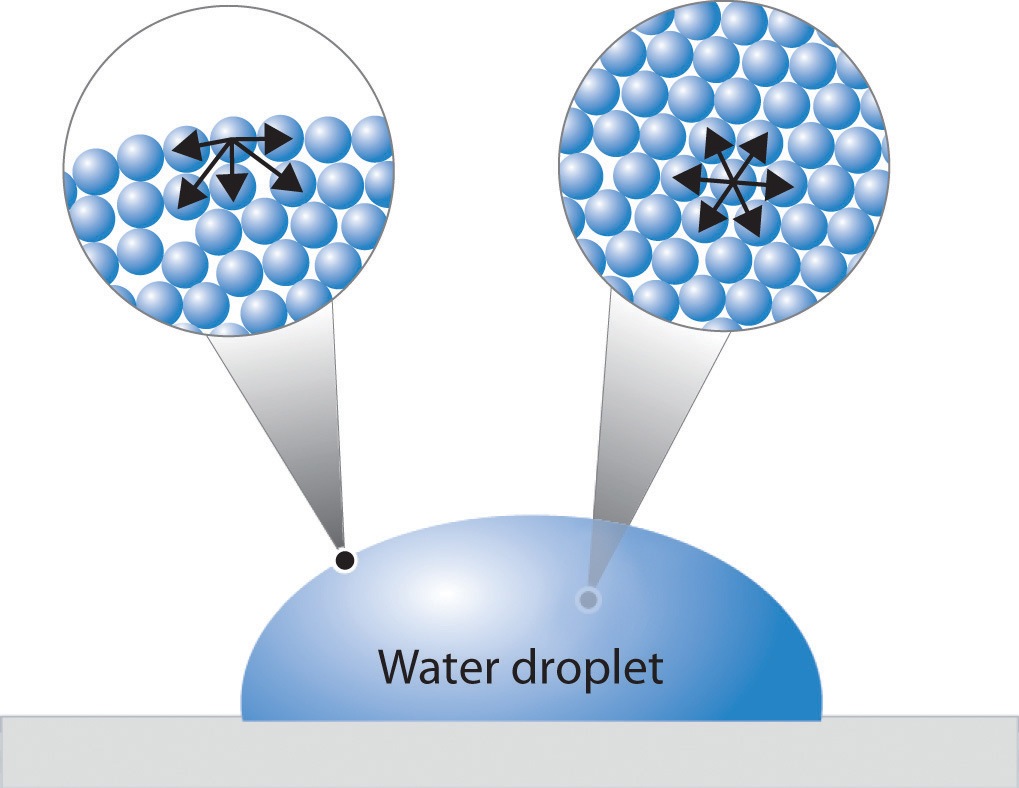
Surface Tension Chemistry LibreTexts

FileSurface Tension Diagram.svg Wikipedia

What is Surface Tension? CTG Technical Blog
Web Surface Tension Is An Important Factor In The Phenomenon Of Capillarity.
It Is Also Responsible For The Beading Up Of Water Droplets On A Freshly Waxed Car Because There Are No Attractions Between The Polar Water Molecules And The Nonpolar Wax.
Web Explore Surface Tension And How It Varies From One Liquid To Another.
Gasoline) Or Solutes In The Liquid (E.g.
Related Post:
